UNIVERSITY OF PISA
“The endocannabinoid system and the de novo ceramide
synthesis pathway as targets for the development of new
anticancer and anti-neurodegenerative agents”
Candidate:
Sara Del Carlo
Tutor:
Dr. Giuseppe Saccomanni
Director of PhD Program:
Prof. Adriano Martinelli
SSD: CHIM/08
School of Graduate Studies
SECRETAZIONE DELL’ ELABORATO SCRITTO
“Il contenuto di questa tesi è strettamente riservato, essendo presenti argomenti tutelati dalla legge come segreti. Pertanto tutti coloro che ne prendono conoscenza sono soggetti all’obbligo, sanzionato anche penalmente dagli articoli 325 e 623 del codice penale, di non divulgare e di non utilizzare
le informazioni acquisite.”
“The content of this thesis is strictly confidential, being protected by the law as secret. Therefore, all those who acquire it are required not to disclose and
not to use the information gained to avoid the sanctions of article 325 e 623 of the criminal code.”
INDEX GENERAL INTRODUCTION
The Endocannabinoid System 1
1.1 Cannabinoid receptors 1
1.2 Endocannabinoid production, activity and hydrolysis. 2
1.3 Targeting CB2 receptor 3
1.3.1 CB2 receptor in the treatment of cancer 3
1.3.2 CB2 receptor in the treatment of neurodegenerative disorders 5
1.3.3 CB2 receptors ligands 7
1.4 Targeting MAGL 8
1.4.1 MAGL inhibition in the treatment of: 9
1.4.2 MAGL inhibitors 9
The Ceramide Pathway 11
2.1 Ceramide production 12
2.2 Ceramide apoptosis induction 13
2.3 Ceramide as biological target 14
Serine palmitoyltransferase (SPT) 15 3.1 Bacterial SPT 15 3.2 Eukaryotic SPT 16 3.3 SPT substrates 17 3.4 Reaction mechanism 17 3.5 SPT inhibitors 20 3.6 Targeting SPT 25
AIM OF THE THESIS 29
SECTION 1. Targeting the endocannabinoid system 33
1.1 Design and synthesis of [18F]-labelled nitrogen heterocyclic derivatives
as new candidate for cannabinoid CB2 receptor PET imaging 35
1.2 Assessment of a high-performance liquid chromatography assay for
human recombinant monoacylglycerol lipase activity 45
SECTION 2. Targeting the de novo ceramide synthesis pathway 2.1 Design, synthesis and biological evaluation of new serine
palmitoyltransferase inhibitors 55
2.2 Development and optimization of an HPLC-FL method to screen
SPT inhibitors 66
2.3 Evaluation and characterization of bacterial SPT inhibitors 74
2.4 An insight into bacterial SPT 87
EXPERIMENTAL PART 93
Introduction
1
The Endocannabinoid System
The endocannabinoid system includes cannabinoid receptors (CB1 and CB2), the endogenous ligands (anandamide and 2-arachidonoyl glycerol), the anandamide transporter protein, and two enzymes responsible for endocannabinoids hydrolysis (fatty acid amide hydrolase or FAAH and monoglyceride lipase or MAGL).
1.1. Cannabinoid receptors
Two cannabinoid receptors have been identified and classified as type 1 (CB1) and type 2 (CB2). These receptors belong to the super family of G-protein-coupled transmembrane receptors (GCPR) and they are characterized by an N-terminal extracellular domain that possesses glycosylation sites, a C-terminal intracellular domain coupled to a G protein complex, and 7 hydrophobic transmembrane segments connected by alternating extracellular and intracellular loops. The human CB1 and CB2 receptors exhibit 68% identity within the transmembrane regions, 44% identity throughout the whole protein. The gene locus for the human CB1 receptor has been localized in chromosome 6 to position 6q14–q15 whereas the gene encoding for human CB2 receptor is located in chromosome 1p36. [1]
CB1 receptor (CB1R) is mainly expressed in the central nervous system and its activation has been associated with most of the psychotropic and behavioral actions of cannabinoid drugs. It has been detected not only in neurones but also in astrocytes and microglial cells. It is also found in a variety of peripheral tissues such as adipose tissue, liver, muscle, the gastrointestinal tract, pancreas, urinary bladder, lung, adrenal gland, testis, ovary, prostate and in rat adipose tissue. [2]
The CB2 receptor (CB2R) is limited essentially to the cells associated with the
immune system, like spleen, thymus, tonsils, B cells and natural killer cells, monocytes, neutrophils and T cells. Although absent from the CNS in normal conditions, CB2 receptors might be induced in brain microglial cells in response to different damaging conditions associated with local inflammatory events. It is also located in retina, skin and some malignant cells. [3]
The affinity of CB1 and CB2 for Gi or Go proteins may be different as
revealed by several studies on cannabinoid ligand binding or regulation of GTPγS binding. Both CB1R and CB2Rare coupled negatively to adenylyl cyclase and positively to mitogen-activated protein (MAP) kinase. Activation of both of them displays a high affinity for Gi, agonist stimulation of CB1 also
result in a high-affinity saturable interaction with Go but CB2 receptors do not
interact efficiently with Go. It has been reported that the affinity of CB1
The Endocannabinoid System
2
1.2 Endocannabinoid production, activity and hydrolysis.
Endocannabinoids are signalling lipid molecules produced on demand by cleavage of membrane phospholipids precursors and their levels are maintained by transport into cells and subsequent intracellular hydrolysis. The most investigated compounds are the partial agonist anandamide (AEA) and the full agonist 2-arachidonylglycerol (2-AG) which are mainly responsible for CB1R and CB2Ractivation, respectively. Anandamide is locally produced by the phospholipase D-mediated cleavage of the membrane precursor called N-arachidonoyl-phosphatidylethanolamine (NAPE) in a Ca2+-dependent manner. On the other side 2-arachidonylglycerol is produced by the diacylglycerols (DAG) lipase cleavage of the membrane precursor. Because endocannabinoids are lipophilic compounds produced from membrane phosphoipids, they do not need to be stored in synaptic vesicles like other neurotransmitters.
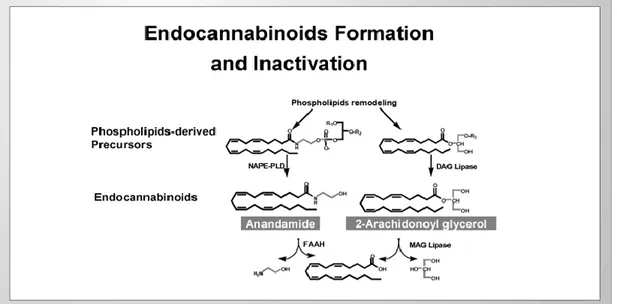
AEA and 2-AG activity is interrupted by carrier mediated cell uptake and subsequent hydrolysis by fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), respectively (Figure 1). These lipophilic ligands are also substrate for other enzymes such as cyclooxygenase-2 (COX-2), different lipoxygenase (LOX) isozymes and cytochrome P450 (CYP2D6). [5, 6]
Figure 1. Most endocannabinoids are long-chain polyunsaturaded fatty acids by-products. Anandamide and 2-arachidonoyl glycerol (2-AG) are produced from the remodeling of phospholipids through pathways that
use NAPE-PLD (N-acylphosphatidylethanolamine-seletive phospholipase D) and DAG (Diacylglycerol) lipase synthesis enzymes. They are rapidly metabolized and hydrolized by FAAH (Fatty Acid Amide
Hydrolase) and MAG L (Monoacyl Gycerol Lipase) enzymes. [7]
More recently, additional endocannabinoid-degrading enzymes were also shown to have key roles in the endocannabinoid system. Two serine
Introduction
3 hydrolases, /β-hydrolase 6 (ABHD6) and /β-hydrolase 12 (ABHD12), were also recently discovered and described as complementary 2-AG-degrading enzymes in the brain. [8, 9] Interestingly, MAGL, ABHD6 and ABHD12 are present in different subcellular locations suggesting distinct roles in controlling 2-AG activities. In addition, another enzyme, called NAcylethanolamine- hydrolyzing Acid Amidase (NAAA), was found to regulate the levels of N-acylethanolamines. [10]
1.3 Targeting CB2 receptor
Today it is well defined that activation of CBRs represents a powerful tool for the treatment of different kind of disease with particular attention to cancer and neurodegenerative disorders. Different synthetic cannabinoid agonists have been developed but in vivo experiments underlined different side effects which are mainly related to stimulation of CB1R. [11] Therefore a lot of drug development strategies has been pursued in order to obtain compounds with a good selectivity toward CB2R avoiding the side effects of CB1R activation.
1.3.1 CB2 receptor in the treatment of cancer
Increased levels of both CB1 and CB2 receptors has been detected in different type of tumour cells. Cannabinoids have shown proliferative, anti-metastatic, anti-angiogenic and pro-apoptotic effects in various cancer types (lung, glioma, thyroid, lymphoma, skin, pancreas, uterus, breast and prostate carcinoma) using both in vitro and in vivo models. [1]
Growth and survival of tumor cells is often dependent on the increased signalling pathways that regulate cell survival and proliferation. Two of these include the MAPK signaling pathway (Ras/Raf – MAPK, extracellular signal regulated kinase (ERK1/2)) and the PI3K/Akt pathway. Both CB1 and CB2 cannabinoid receptors are coupled to these pathways via heterotrimeric Gi/o-proteins. The molecular mechanisms by which cannabinoid receptors modulate these mitogenic signaling pathways are not yet fully understood. One theory for modulation of the PI3K/Akt pathway by cannabinoids involves the CB1/CB2 linked G-protein βγ. Other theories for the modulation of the MAPK pathway by cannabinoids involve tumor necrosis factor (TNF) and ceramide synthesis. More support for the role of TNF- has come from a recent report using specific CB1R and CB2R agonists in both in vitro and in
vivo models of colon cancer. [1, 4] Specifically, cannabinoid receptor
activation stimulated a 4-fold-increase in TNF- production in DLD-1 and HT29 colon cancer cells and further experiments demonstrated that TNF- was the main mediator of ceramide de novo synthesis. Therefore, in models of
in vitro and in vivo colon cancer, ceramide is an important mediator of the
The Endocannabinoid System
4
in colon tumors, specific ligands for CB2 may prove to be valuable adjuvants to colon cancer chemotherapy. [12]
JWH-133 is a synthetic selective cannabinoid ligand for the CB2 receptor with 200-fold higher affinity for the CB2 (Ki = 3.4 nM) over the CB1 receptor (Ki = 677 nM). JWH-133 has been tested in different type of in vivo and in vitro experiments to evaluate the anti-tumor activity in absence of CB1R side effects. JWH-133-mediated decrease in cell viability was due to an induction of apoptosis via ceramide synthesis and ERK1/2 activation. Investigation in a mouse model of glioma showed that JWH-133 caused a CB2 receptor mediated 71% decrease of tumor growth and completely inhibited the growth of highly malignant (grade IV) human astrocytomas in the same mouse model. [13] The anti-tumor effect observed was mediated by induction of apoptosis via ceramide synthesis and ERK1/2 activation. JWH-133 has also shown anti-cancer effects in in vivo skin tumor models. Treatment of skin and breast cancer cell lines with JWH-133 led to growth and migration reduction of tumor cells. [5] Moreover in vivo treatment of pancreatic tumor cells with THC and the CB2- selective agonist JWH-133 causes a notably reduction of pancreatic tumor growth and cells spreading. [14]
Moreover it was demonstrated that activation of CB2 receptor in mantle cell lymphoma induces apoptosis via a sequence of events represented by: accumulation of ceramide, phosphorylation of p38, depolarization of the mitochondrial membrane, and caspase activation. [15] Investigation on CB2-receptor mediated apoptosis in human leukemia cells demonstrated that receptor activation stimulates serine palmitoyltransferase (SPT) causing accumulation of de novo synthesized ceramide which mediates loss of mitochondrial membrane potential, citocromo C release and caspase activation. [16]
In addition to their direct anti-proliferative and pro-apoptotic effects on tumor growth in vitro and in vivo, cannabinoid-related drugs have been reported to affect tumor progression through the inhibition of key events: cell migration, invasion, metastasis and tumor neo-angiogenesis could be inhibited in vivo both indirectly through an inhibition of the angiogenic factors produced by tumor cells and directly through an action on endothelium (Figure 2) [4, 5]
Introduction
5
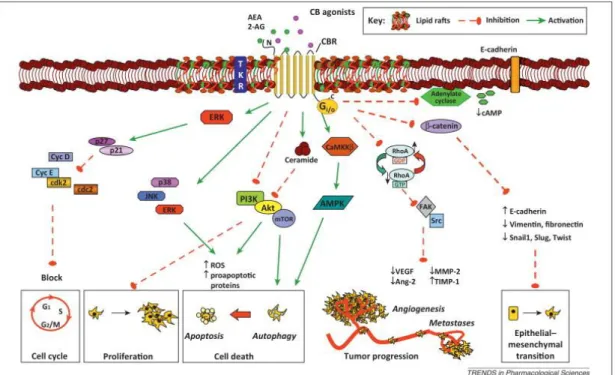
Figure 2. Highlighted pathways of the antitumor effects of cannabinoid agonists in cancer. The figure depicts the main signaling cascades elicited downstream of CB receptor activation by endocannabinoids and
cannabinoids, which affect all the hallmarks of cancer: inhibition of cell proliferation; cell-cycle arrest; induction of cell death (apoptosis and autophagy); prevention of tumor progression (cancer cell vascular adhesiveness, invasiveness, and metastasis formation); inhibition of angiogenesis in tumor environment; and
inhibition of the epithelial–mesenchymal transition. [1]
1.3.2 CB2 receptor in the treatment of neurodegenerative disorders
CB2R plays an important role in neuroinflammation and neurogenesis therefore targeting this receptor in neurodegenerative diseases might be an useful therapeutic approach. Conversely CB1R mediates undesired side effects that can be related to its psychoactive activity.
Resident immune and CNS cells express functional CB2 receptors whose activation results in the modulation of the inflammatory response, restraining one of the agents responsible for the demyelination progression and neuronal death. The modulation of inflammatory molecules mediated by CB2 receptors could also enhance remyelination, stimulating the survival of oligodendrocyte precursors and neural stem/precursor cells, and their development into mature oligodendrocytes. However, the role of CB2R in controlling demyelination and enhancing remyelination is not limited to autoimmune diseases and it is not restricted to the control of the immune system. Both in multiple sclerosis and other non-immunomediated demyelinating diseases, the protective effect of CB2R agonists on neural cells is a remarkable advantage. Moreover, CB2 receptor activation may be a relevant strategy in cellular replacement. However, before proposing the usefulness of CB2 agonists for myelin disorders, it is necessary to obtain a deeper understanding of what effects may
The Endocannabinoid System
6
be attributable to the activation of CB2 receptors alone, and which are also due to the participation of the CB1 receptor. Given current understanding of CB2 receptors and the pathogenesis of immune-mediated or other demyelinating disorders, these receptors seem to be of potential therapeutic interest.
Alzheimer’s disease
Alzheimer’s disease (AD) accounts for the most frequent form of dementia in the elderly and is characterized by progressive deterioration of cognition and memory. This pathologies is defined by neurofibrillary tangles, amyloid-beta (Aβ) plaques and synaptic degeneration. Amyloid plaques accumulation triggers their precipitation leading to the formation of intraneuronal ‘neurofibrillary tangles’. Activated microglia and astroglial cells attempt to encapsulate and degrade the amyloid deposit causing a massive local accumulation of inflammatory cytokines and reactive oxygen species (‘cytokine cycle’).
The analysis of human post-mortem brain samples from AD patients highlighted that Aβ deposition induces upregulation of CB2R and FAAH restricted to microglia and astrocytes respectively. Moreover different studies demonstrate that activation of CB2R reduces microglia activation and inhibit TNF- and nitric oxide production suggesting that upregulation of this receptor might be a neuroprotective response to neuroinflammation. [17, 18] Multiple sclerosis
Multiple sclerosis (MS) is a immune-mediated neuroinflammatory diseases characterized by immune response towards myelin self-peptides which leads to myelin sheathes disruption. Its aetiology is unknown but much evidence suggests that genetic and environmental factors may have an important role on multiple sclerosis (MS) susceptibility, although the possibility of a role for infectious agents has also been considered. Its characteristic symptoms (such as painful muscle spasms, tremor, ataxia, weakness or paralysis) are thought to be the result of new lesions and expansion of old lesions at the CNS level. Since there is no cure for MS, any treatment is only a palliative resource. New evidence suggests that the CB2 receptor could also be a pharmacological target, as its expression is increased in several cell types directly involved in the pathogenesis of MS: inhibition of microglia activity, by the activation of CB2 receptors reduces MS progression in animal models. [19]
Huntington’s disease
Huntington's Disease (HD) is a brain disorder that affects a person's ability to think, talk, and move. In Huntington's disease, neurodegeneration of striatal medium-sized spiny neurons and unbalanced neurotransmission are largely attributed to the interference of mutant huntingtin with transcriptional
Introduction
7 regulation. Activated microglia has been identified in HD patients and positively related to HD severity. Some studies demonstrate that CB2 receptor activation is neuroprotective in Huntington's disease models by controlling deleterious microglial activity. [19, 20]
Amyotrophic Lateral Sclerosis (ALS)
ALS is a fatal neurodegenerative disease that primarily affects motor neurons in the spinal cord, brainstem, and motor cortex, leading to complete paralysis. As well as for AD, ALS involves inflammatory processes that play an important role in disease progression. Administration of a selective CB2 agonist (AM1241) in an ALS mouse model (hSOD1(G93A) transgenic mice) delayed loss of motor function and paralysis suggesting a cannabinoid CB2-mediated modification of disease progression. [21]
1.3.3 CB2 receptors ligands
Different compounds able to interact with the CB2 receptor has been developed. These molecules can be divided into non selective CB1/CB2 agonists and CB2 selective ligands.(Figure 3) Among the first group of drugs, dronabinol and nabilone has been licensed for clinical use as antiemetics. In addition, a medicine that contains 9-THC and cannabidiol (Sativex), recently has been licensed for the treatment of neuropathic pain associated with multiple sclerosis. [22] Focusing the research on CB2 selective ligands to avoid undesired psychotropic effects different chemical scaffolds has been developed by both industry and university research laboratories identifying both full, partial and inverse agonists and antagonist ligands whose therapeutical relevance is under evaluation. [23, 2]
The Endocannabinoid System
8
1.4 Targeting MAGL
MAGL is a serine hydrolase that preferentially hydrolyzes monoacylglycerols to glycerol and fatty acid thanks to the catalytic triad Ser-Hist-Asp. This enzyme belongs to the /β hydrolase family and it’s expressed in brain, white adipose tissue, muscle, kidney, ovary, testis and liver. [1] This soluble enzyme is associated with membranes and is involved in both endocannabinoid and eicosanoid pathway. In fact 2-AG is hydrolyzed to arachidonic acid (AA) which represent the precursor of pro-inflammatory eicosanoids. 2-AG hydrolysis is also performed by phospholipase A2 predominantly in gut, spleen and macrophages, whereas MAGL activity is greater in brain, liver and gut. [6] MAGL is expressed pre-synaptically because of 2-AG is synthesized in postsynaptic neurons to interact with the presynaptic CB1R. Inhibition of MAGL activity causes an increase of 2-AG and therefore an increase of CB receptor stimulation avoiding a full-blown cannabinoid-behaviors. Moreover the reduced 2-AG hydrolysis lowers the eicosanoid lipid signalling pathway.(Figure 4)
Figure 4. MAGL coordinately regulates multiple lipid signaling pathways. MAGL blockade leads to an accumulation of the endocannabinoid 2-AG to enhance signaling upon cannabinoid receptors CB1 and CB2.
In certain tissues, such as the brain, liver, and lung, MAGL controls the primary AA precursor pool for pro-inflammatory prostaglandin production. Blocking MAGL thus leads to a variety of beneficial effects through
either enhancing endocannabinoid signaling or suppressing eicosanoid production. In cancer, MAGL plays a distinct role in controlling global FFAs levels that serve as the building blocks for synthesis of pro-tumorigenic signaling lipids such as PGE2 and lysophosphatidic acid (LPA). Blocking MAGL in aggressive
Introduction
9
1.4.1 MAGL inhibition in the treatment of:
Pain
Preclinical studies suggested that MAGL inhibition could represent an interesting strategy for treating pain. [24] Inhibition of 2-AG hydrolysis allowed to obtain an activation of CB1 receptor involved in the antinociceptive action and to reduce inflammatory response by CBRs activation and by lowering prostaglandins and thromboxanes.
Neurodegenerative diseases
Inhibition of MAGL in Parkinson’s disease model by the selective inhibitor JZL184 prevents dopaminergic neurodegeneration, dopamine loss and reduces pro-inflammatory eicosanoids. A similar phenomenon was observed in AD mouse model where a reduction of astrocytes and microglia activation led to reduction of β-amyloid plaques.
Cancer
CBRs activation induces anti-cancer effects through in vitro apoptosis induction and in vivo reduction of angiogenesis and metastasis. [4] COX2-mediated prostaglandin production has been implicated in cancer progression. Genetic or pharmacologic inactivation of COX has been shown to curb cancer malignancy. Therefore inhibition of MAGL represent a dual approach in cancer therapy.
MAGL is upregulated in aggressive human cancer cells and primary tumors where it has a unique role of providing lipolytic sources of free fatty acids (FFAs) for synthesis of oncogenic signaling lipids that promote cancer aggressiveness. Aggressive breast, ovarian, and melanoma cancer cells migration, invasiveness, and tumorigenicity are reduced by MAGL inhibition through lowering FFAs and protumorigenic signaling lipids, which includes lysophosphatidic acid and prostaglandins. [25] Moreover enhancement of anti-tumorigenic cannabinoid pathways by MAGL inhibitors impairs prostate cancer pathogenicity and colorectal cancer tumorigenesis. [26, 6]
1.4.2 MAGL inhibitors
MAGL inhibitors might interact with the enzyme in a reversible or irreversible manner. Generally they are classified in relation to their chemical structure and mechanism of interaction in:
General serine hydrolase inhibitors.
These compounds are characterized by a common mechanism of interaction with the serine group involved in the catalytic triade leading to a non selective inhibition of both FAAH and MAGL. Three main reactive groups can be distinguished (fluorophosphonates, trifluoromethylketones and sulfonylfluorides) whose representative
The Endocannabinoid System
10
drugs are methyl arachidonylfluorophosphonate (MAFP),
arachidonyltrifluoromethylketone (ATFMK) and
phenylmethylsulfonylfluoride (PMSF). Arachidonoylglycerol analogs.
Compounds inspired to the endogenous ligand 2AG has been developed by modifications of the fatty acid chain, linker and glycerol moiety.
De novo inhibitors.
The two main selective MAGL reference inhibitors belonging to this class of compounds are URB602 (IC50= 28-75 μM depending on the
source of enzyme ) and JZL 184 (IC50=9.6-17 nM). The first molecule is characterized by a partially reversible mechanism of interaction whereas JZL184 causes an irreversible inhibition of the enzyme. [27]
Inhibitors targeting the essential sulfhydryl group(s) of MGL
Irreversible inhibitors of MAGL has been developed functionalizing the reactive structure of maleimide: this molecules is able to react with cystein residues close to the catalytic pocket leading to a new covalent bond hindering the enzyme-substrate interaction.
Introduction
11
The Ceramide Pathway
Cells survival or death is a process finely tuned by different signaling molecules. Two different mechanisms lead to cells death: necrosis and apoptosis. The first pathway involves a non-programmed death of cells that occurs after negative stimuli from the outside causing cell membrane break and release of its contents in the environment. This phenomenon causes an inflammatory response and neighbor cells death. On the contrary apoptosis is a form of “cell suicide” which involves an organized death of a single cell avoiding inflammatory response: a shrinking of cells take place to disconnected the suicide cells from the others and nucleic DNA condenses and marginates at nuclear membrane. Then the cell is divided into few “apoptotic bodies” which are engulfed by macrophages.
The terms apoptosis represent a natural and vital process which plays an important role in the development of multicellular organisms and in the regulation and maintenance of the cell populations in tissues upon physiological and pathological conditions. Dysfunction or dysregulation of the apoptotic program is implicated in a variety of pathological conditions: a reduction of apoptosis can result in cancer, autoimmune diseases and spreading of viral infections, while neurodegenerative disorders, AIDS and ischemic diseases are caused or enhanced by excessive apoptosis. [28]
Different biological pathway are involved in apoptosis regulation and an important effector is represented by a sphingoide base called ceramide. This molecule is ubiquitously distributed into cells determining protection and cell sustenance at low concentrations and death threat when over produced. Therefore modulation of ceramide levels has an important role on cells signalling networks.
Ceramides are sphingolipids constituted by a sphingoid base linked trough an ammidic bond to fatty acid acyl chain. They can be classified in relation to the sphingoid base, fatty acid and the polar head. Ceramide contains sphingosine which has a trans-double bond at the C4-5 position in the sphingoid base backbone. (Figure ) Functionalization of hydroxyl group in position C1 with phosphocoline or one (or more) sugar leads to phospholipids and glycosphingolipids respectively. [29]
Ceramide
12
2.1 Ceramide production
Different pathways to produce ceramide are located in various part of the cell in relation to sphingolipids main role in cell life and to their hydrophobic properties. These biosynthetic cascades differ by the starting material used: the anabolic pathway is called de novo ceramide synthesis and involves the use of L-serine and palmitoyl-CoA as starting material for the synthesis of ceramide. This biosynthetic cascade takes place in the endoplasmatic reticulum (ER). On the contrary the catabolic pathway involves sphingomyeline (SM) phosphodiester bond hydrolysis by sphingomyelinases (SMase) leading to ceramide and phosphorylcholine. The activation of different catabolic enzymes yields ceramide within a few minutes whereas the
de novo synthesis produces ceramide in several hours. Another way to
produce ceramide involves the recycling of catabolic fragments generated by ceramide hydrolysis thanks to the reverse action of ceramidases (CDase), the enzyme responsible of ceramide hydrolysis. This route is called the “salvage pathway”. (Figure ) [29, 30]
Figure 6 [30]
De novo ceramide synthesis:
The de novo synthetic pathway of ceramide takes place at the citoplasmatic face of smooth ER. Serine palmitoyltransferase (SPT) catalyses the condensation reaction of L-serine and palmitoyl-CoA leading to 3-ketodihydrosphingosine (or 3-ketosphinganine) which is reduced to sphinganine. Then this sphingoid base is acylated by dihydro-ceramidase synthase (CerS) to dihydroceramide that is finally converted to ceramide by dihydro-ceramidase desaturase.
Catabolic pathway of ceramide production:
Sphingomyelin is a phosphosphingolipid which has an important role in cellular membrane bilayer. Hydrolysis of this molecule by SMase represent a
Introduction
13 fast way to obtain ceramide. The peculiarity of this catabolic pathway is done by different isoform of SMases which are classified by their pH optimum, subcellular distribution and regulation. Acidic sphingomyelinase (aSMase) is located in lysosomes and explicates its optimal enzymatic activity at pH 4.5-5 and it is required for cellular membrane turnover. Neutral sphingomyelinase (nSMase) is a membrane bound enzyme expressed in ER and Golgi membrane and in particular region of the body such as kidney and brain.
Salvage pathway:
Ceramide levels are endogenously regulated by both production and degradation. This last event is generated by the hydrolytic activity of CDase in lysosomes leading to sphingosine which is able to move in the ER. The catabolic fragments generated by CDase can be recycled thanks to the reverse action of this enzyme which catalyses the condensation of sphingosine and a fatty acyl-CoA in ER. Different isoforms of CDase have been identified: acidic CDase (aCDase) is located in lysosomes and neutral/alkaline CDases in mitochondria and nuclear membrane where they are responsible of ceramide generation.
2.2 Ceramide apoptosis induction
Ceramide apoptosis induction can occur by sphingomyeline hydrolysis or by
de novo ceramide synthesis that take place in different sites of the cell.
The downstream target involved in the de novo ceramide apoptosis induction is largely unknown.
On the other hand sphingomyeline hydrolysis cascade has been better defined. SMase activation occurs in response to stimulation of cell surface receptors of the tumor necrosis factor (TNF) upon the binding with specific ligands such as TNF alpha, TNF-related apoptosis-inducing ligand (TRAIL) and Fas ligands. SM hydrolysis in response to TNF signals involves both nSMase and aSMase but their activation occurs through different mechanisms.
Activated aSMase translocates from its intracellular locations to the plasma membrane where it reaches SM. The ceramide produced by aSMase activates the aspartyl protease cathepsin D that can subsequently cleave the pro-apoptotic Bcl-2 family member Bid. Activation of Bid induces cytochrome C release from mitochondria and activation of caspase-9 and -3, leading to apoptotic cell death by the intrinsic pathway.
Ceramide generated by nSMase leads to activation of ceramide-activated protein kinase (CAPK) and ceramide-activated protein phosphatases (CAPPs), direct downstream targets of ceramide. CAPK, a Ser/Thr protein kinase, is involved in the mitogen activated protein kinase (MAPK) cascades
Ceramide
14
that induce the extracellular-signal regulated kinases (ERK) activation. ERK cascade leads to cell cycle arrest and cell death. [29]
Different stimuli are responsible for activation of catabolic or anabolic ceramide production even if some agents are able to induce both pathway. TNF-related apoptosis inducing ligands and the DNA-damaging drug doxorubicin are able to activate both SMase and de novo pathway whereas other substances such as retinoic acid and ethanol are able to stimulate de
novo route or SMase respectively.
2.3 Ceramide as biological target
Modulation of ceramide levels and therefore of apoptotic events represent a therapeutical tool against cancer and neurodegenerative diseases. An increase of ceramide levels induce one or more antiproliferative responses involving either cell-cycle arrest, apoptosis, senescence and/or differentiation. [31] Unfortunately natural ceramide is unable to cross the cell membrane and, therefore, cannot be used directly for therapeutic purposes. For this reason, ceramide analogs able to pass the cell membrane and then to mimic the effects of endogenous ceramide has been developed. [32]
Regulation of ceramide is also obtained by direct interference on biological pathway responsible of its production or degradation but also having effect on others endogenous system which are able to stimulate/inhibit ceramide mediated apoptosis. Radiation and chemotherapic agents such as cisplatin and campothecin increase ceramide levels enhancing sphingomyelin hydrolysis whereas daunorubicin, irinotecan and gemcitabine stimulate de novo synthesis as well as cannabinoids (Figure 7). [31, 33]
Introduction
15
Serine palmitoyltransferase (SPT)
Sphingolipids biosynthesis differs between species whereas the first enzymatic step of de novo biosynthesis is conserved across all species producing sphingolipids. The common step of the de novo pathway is the enzymatic condensation carried out by serine palmitoyltransferase (SPT), a pyridoxal-5’-phosphate (PLP) dependent enzyme belonging to alfa-oxoamine synthase (AOS) family. [34]
SPT catalyzes a condensation between L-serine and typically C16 acyl-CoA thioester (palmitoyl-CoA), to give a Claisen-like C18 condensation product: 3-ketodihydrosphingosine (KDS). (Figure 8) SPT plays a key role in the biosynthesis of sphingolipids indeed regulation of SPT catalyzed step prevents accumulation of sphingolipids metabolites (i.e. sphingoid base) while inhibition of later biosynthetic steps leads to metabolite accumulation which are death effectors in various experimental models and pathological conditions. Therefore, SPT condensation reactions represent the rate-limiting step of sphingolipids biosynthesis. [35]
Figure 8. Reaction catalyzed by serine palmitoyltransferase [36]
3.1 Bacterial SPT
The first three dimensional structure of SPT was obtained by the isolation of the homodimeric (and water soluble) SPT from S. paucimobilis (spSPT). [37] The crystal structure revealed that the holo-SPT monomer consists of three domains: the N-terminal, the central catalytic domain and the C-terminal domain. The N-terminal domain consists of 80 residues (an α-helix followed by 3 β-sheets) and is linked with the central domain also called catalytic domain. The central domain is composed by 7 β-sheet (about 200 residues) where is located the Lys265 residue responsible of PLP cofactor binding. The C-terminal domain is strictly linked with N-terminal domain by binding interactions. [34] For the correct positioning of the PLP cofactor (crucial for the catalytic activity) π-stacking interactions between the pyridine ring and His159 are required. PLP interacts also with other residues such as Asn138, Asp231, His234, Thr262, Gly134 and Tyr135. Furthermore, an Arg379 has a crucial interaction with the carboxy moiety of the substrate which is necessary for the positioning of the L-serine in the catalytic site.
Other SPT homologues, like Sphingobacterium multivorum SPT (SmSPT) and Sphingobacterium wittichii (SwSPT), exhibit differences at few levels. SmSPT have a 70% sequence identity and the external aldimine (formed by
Serine palmitoyltransferase
16
the PLP and serine) is linked by two water molecules to two aminoacid residues, Ser81 and Met271, and (not to a Lys) in active site. [38] Three-dimensional structure of SwSPT revealed a larger active site than in SpSPT, probably because of the use of a larger acylated-ACP thioester substrate. [39]
3.2 Eukaryotic SPT
Eukaryotic SPT is a heterodimer consisting in two subunits, LCB1 and LCB2, both linked to the endoplasmatic reticulum (ER). They are encoded by two different genes, lcb1 (SPTLC1) and lcb2 (SPTLC2), composed by 15 exons of 85 kbp size localized on chromosome 9, arm q21-q22, and 12 exons of 110 kbp size localized on chromosome 14, arm q24.3-q3 respectively. [35] These genes encode for proteins of 53 and 63 kDa, with 20 % sequence identity that is probably critical for dimerization. LCB proteins have 95% identity between mammals and 40% between mammals and yeast. The sequencing of SPTLC1 and SPTLC2 genes in Saccharamyces cerevisiae revealed that the active site of eukaryotic SPT is localized in the LCB2 subunit; this observation is suggested by detection of conserved residues of AOS’s family (one lysine, two hystidine and an aspartate) which are essential to lock the PLP cofactor in the active site. [34, 35] LCB1 subunit appears crucial for SPT’s catalytic activity. The lack of this subunit expression or a missense mutation in SPTLC1 gene causes alteration of SPT’s catalytic activity. Furthermore, CHO cell line defective in SPTLC1 transcription expresses lower levels of LCB2. Moreover LCB2 appears to be unstable when it is not associated with the LCB1. On contrary an high levels of LCB1 do not require an overexpression of LCB2. Both monomers have only one highly hydrophobic transmembrane domain (TMD). The catalytic site has a cytosolic orientation indeed indirect immunocytochemical analysis indicated that C-termini and N-termini of LCB1 have cytosol and lumen orientation respectively. Another isoform of LCB2 called LCB3 is expressed only in certain tissues.
In yeast this enzyme is constituted by the so called “SPOTS COMPLEX” that’s encodes for SPT–ORM1/2-Tsc3-Sac1 (phosphatase). Therefore, LCB1 and LCB2 subunits are associated with a third subunit, Tsc3, which is required for the maximal SPT activity. Moreover this heterodimeric structure is associated with oromucosoids proteins (ORM1/2) which are able to negatively regulate SPT. [39]
Recently for human SPT two novel small subunits named ssSPTa and ssSPTb were discovered. These subunits can enhance activity >10 fold when bound to LCB1-LCB2 heterodimer. Moreover orosomucoid-like (ORMDL) proteins appear to be able to interact with the LCB1-LCB2 heterodimer. [40, 41]
Introduction
17
3.3 SPT substrates
Palmitoyl-CoA is the more kindred acyl-CoA thioester substrate of SPT in mammalian cells; on the other hand pentadecanoyl-CoA and heptadecanoyl-CoA are also good substrates less abundant. L- and D- serine are SPT amino acid substrates. SPT is able to form the Schiff’s bases with both enantiomers but the alfa-deprotonation step (before acylation) proceeds only with L-serine. Thus L-serine is the common SPT’s substrate in the de novo synthesis. The structural analogues of L-serine (serinamide, serinol, serine methyl ester) were also tested as substrate indicating that the carboxyl groups of L-serine are responsible for the recognition of the amino acid substrate by the SPT enzyme. [37]
3.4 Reaction mechanism
SPT catalyzes a Claisen like condensation of L-serine and palmitoyl-CoA which leads to 3-KDS as final product. SPT reaction mechanism proceeds across six step: 1) formation of the Schiff base between L-serine and PLP, 2) L-serine α-hydrogen removing; 3) nucleophilic attack to palmitoyl-CoA (formation a transient acylated adduct), 4) decarboxylation, 5) protonation of α-carbanion and 6) KDS release (Figure 9). Assays conducted with purified enzyme from chinese hamster ovary cells, suggested that one molecule of mammalian SPT is capable of catalyzing maximally of 80 cycles of these steps per minute. [35]
Serine palmitoyltransferase
18
Studies on bacterial form of SPT allowed to investigate the intermediates involved in the catalytic cycle. The PLP cofactor catalyses all the reaction mechanism. Interaction of PLP aldehyde group and Lys265 leads to a Schiff base (internal aldimine) in the active site. Spectrophotometric analysis of holo spSPT revealed two UV absorption peaks which represent two tautomers of the Schiff base PLP-Lys265: the enolamine (338 nm) and ketoenamine (426 nm). Moreover, crystalline structure of SmSPT and spSPT highlighted that PLP has Van der Walls interaction with His159 and Ala233. Addiction of L-serine to the holo-enzyme causes drastically changes in the UV spectra due to the formation of a new Schiff base between PLP and L-serine (external aldimine). Two intermediate adducts are identified: first adduct has an absorption similar to holo-SPT while the other one has a maximum peak at 426 nm (which represent the ketoenaminic tautomer of the new Schiff base) (Figure 10). The interaction of PLP with L-serine causes a rotation of pyridine ring or a torsion of the Schiff base C4-C4’ bound.
Figure 10. Spectrophotometric analysis of holo SPT[42]
Once the external aldimine is formed, L-serine is conformationally stabilized by interaction of the carboxyl group with His159 Nε2 (hydrogen bond) and by interaction of the hydroxylic group with the PLP phosphate group and a molecule of water. These interactions fix the PLP-L-serine complex allowing a perpendicular orientation (80°) of Cα-COO
bond to imine-pyridine plane. [34, 43]
The next step of the reaction mechanism is represented by the formation of a new C-C bond via a Claisen like condensation. Two different intermediates might be involved in the nucleophilic substitution: the decarboxylated intermediate or that one generated after removal of the -H. In order to understand the intermediate involved in the nucleophilic substitution reaction some experiments have been developed in presence of the palmitoyl-CoA analogue S-(2-oxoheptadecyl)-CoA. This compound has a methylene group
Introduction
19 inserted between the sulphur atom and the carbonyl groups which doesn’t allow the nucleophilic substitution. Data obtained performing the enzymatic reaction in presence of S-(2-oxoheptadecyl)-CoA and deuterated solvent showed that the α-hydrogen switch with the deuterated solvent increases of about 100 times. Therefore, α-deprotonation of external aldimine is improved in presence of palmitoyl-CoA substrate and a significative amount of quinonoid adduct have been formed. [44] This suggests that the quinonoid adduct attacks palmitoyl-CoA thioester to form the C-C bond. [44] A faster α-deprotonation of external aldimine in presence of palmitoyl-CoA reduce the risk of intermediates accumulation which can form pyridoxamine-5’-phosphate (PMP) after transamination reaction (PMP can’t be converted to PLP thereby the enzyme is inactivated). According to Dunathan hypothesis, the -deprotonation process takes place when the bond involved in the nucleophilic substitution is perpendicular to the imine-pyridine thanks to the complete overlap between the -orbital (occupied) of the designed bond and the free -orbital of the conjugate imine-pyridine system. [45] The increase of -deprotonation process in presence of palmitoyl-CoA is related to a strictly stereochemical control of the reaction. In the external aldimine conformation the C -H bond is 40° rotated with respect to the imine-pyridine plane avoiding the -deprotonation process. [44] This conformation is generated by interaction of the carboxyl group of L-serine with His159 as observed in the SPT-PLP-serine crystal structure. A computational study showed that palmitoyl-CoA induced conformational change is related to the displacement of the hydrogen interaction of L-serine with His159: palmitoyl-CoA interacts with His159 (hydrogen bond) whereas L-serine performs a new interaction with the guanidine group of Arg390 rotating the C -N bond of 50°. In this new conformation the C -H bond of L-serine is perpendicular to the imine-pyridine plane promoting the -deprotonation. [46] The nucleophilic substitution of quinonoid intermediate to the acyl-CoA thioester carboxylic group leads to a β-keto-acid derivatives. The interaction between palmitoyl-CoA and His159 promotes the C-C bond formation by acidic catalysis played by His159: this residue exchange an hydrogen with the carboxylic group of palmitoyl-CoA inducing the right positioning. The product forms an hydrogen bond with His159 (the oxygen of the carbonyl group interact with the hydrogen of His159). Then the β-keto-acid derivatives undergoes to decarboxylation and release of the final adduct (KDS).
Generally PLP catalyzed reactions occur through decarboxylation process catalyzed by the electron withdrawing immine-pyridine system. Instead SPT decarboxylation reaction is promoted by the correct position of the carboxyl group by His159.[35, 43]
Serine palmitoyltransferase
20
3.5 SPT inhibitors
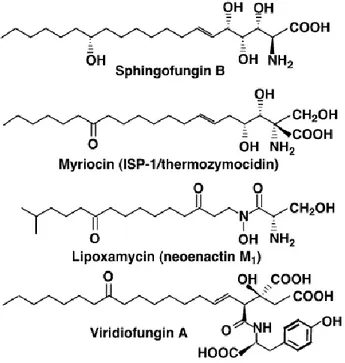
Natural inhibitors of SPT have been discovered. Sphingofungine, lipoxamicina (neoenactin M1), and myriocin (ISP-1/thermozymocidin) are potent and highly selective inhibitors of both fungine and mammals SPT in cell-free models with nanomolar IC50. (Figure 11)
The inhibitory activity of sphingofungine B is highly dependent on stereochemistry: the C14 hydroxyl group of sphingofungine B yields potent inhibitory activity but it’s not crucial for the activity. On the other hand, the configuration of the stereogenic centers in the α, β, γ and δ positions from carbonylic group are essential for the inhibitory activity. [47]
Viridiofungins were first isolated by Harris and co-workers in 1993 from the fungus, Trichoderma viride. This family of alkyl citrates exhibited broad spectrum of anti-fungal properties with minimum fungicidal concentrations in the range of 1–20 μg/mL against a number of species. Furthermore, viridiofungins inhibited rat and yeast squalene synthesis and showed very potent (nanomolar range) inhibitory activity against serine palmitoyltransferase.
Figure 11. Structure of natural SPT inhibitors.
L- Penicillamine and L-cysteine
Penicillamine (Pen) is an α-amino acid and a characteristic degradation product of penicillins and the enantiomer used as pharmaceutical drug is D-Pen. It has been well established that both enantiomers and racemic mixture exerts anti-PLP activity but L-Pen has the stronger inhibitory activity thanks
Introduction
21 to the structure similarity with the enzyme’s naturally substrate (L-amino acids) (Figure 12).
Figure 12. L-amino acids inhibitors of SPT.
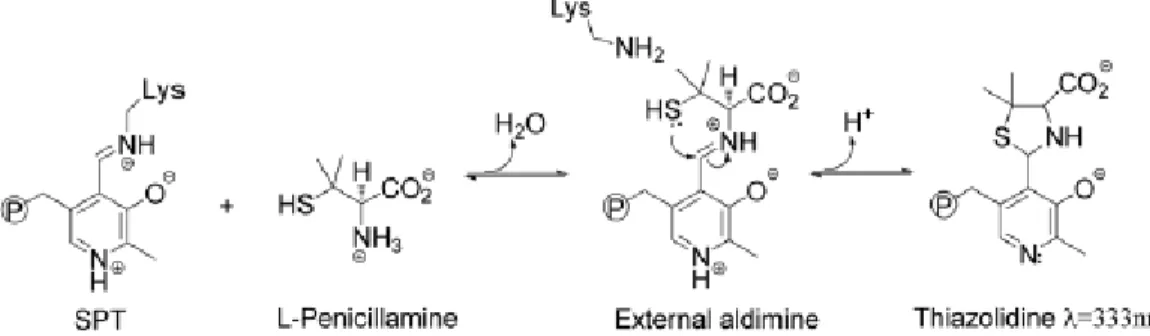
Incubation of D-Pen and L-Pen with spSPT results in different percentage of enzyme inhibition: incubation of 5 mM L-Pen with spSPT reduces enzyme activity to 3% while incubation with D-Pen decreases enzyme activity to 34%. Dialysis of enzyme inactivated by D-Pen and L-Pen with a buffer containing 50 µM of PLP restores SPT activity demonstrating that Pen inhibition is reversible and the mechanism of SPT inactivation occurs by disabling the PLP cofactor. The Pen thiol-group is more nucleophilic than the hydroxyl group of the L-Ser and it’s able to interact with PLP aldehyde leading to PLP:thiazolidine (PLP:TA) adduct (Figure 13) whose structure was confirmed by ESI-MS analysis.
Figure 13. Addition of L-Pen to SPT leads to formation of a PLP:TA adduct via an external aldimine intermediate (P represents group phosphate). [48]
Moreover L-Pen and L-cystein activities have been compared highlighting a faster inhibitory activity of L-Pen that could be related to the presence of
gem-dimethyl group enhancing cycle formation through a Thorpe-Ingold
effect. [48]
β-chloro-L-alanine and Cycloserine
β-chloro-L-alanine (BCA) and L-cycloserine has been used as SPT inhibitors. These compounds are potent inhibitors of several PLP-dependent enzymes, therefore their use as specific inhibitors is limited. [49]
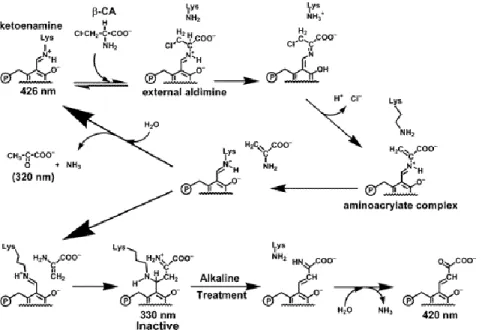
BCA is a suicide inhibitor of SPT which irreversibly inactivates the enzyme leading to the formation of a PLP-adduct and pyruvate. (Figure 14) This mechanism of inactivation is catalyzed by deprotonation of the C of BCA and. after elimination of the chloride ion an aminoacrylate complex is formed. This intermediate regenerates the internal aldimine releasing aminoacrylate
Serine palmitoyltransferase
22
which is involved in two different pathways: the hydrolysis to pyruvate and ammonia or the nucleophilic attack to the internal aldimine leading to the irreversible inhibition of SPT. [36]
Figure 14. Proposed Reaction Mechanism of SPT with βCA, Based on the Spectral Characteristics of the PLP-adduct: the external aldimine intermediate is formed between βCA and the PLP of SPT. After the
α-proton of βCA is subtracted by Lys265 of SPT, elimination of the chloride ion occurs to form the aminoacrylate-PLP aldimine. Aminoacrylate is released and the internal aldimine is regenerated by
transaldimination. [36]
Cycloserine is cyclic α-aminoacid able to inhibit many PLP-dependent enzymes (transaminase, racemase and decarboxylase). It exists as two enantiomers: D-cycloserine (DCS) and L-cycloserine (LCS) (Figure 15). DCS is an agonist of N-methyl-D-aspartic acid (NMDA) receptors that is implicated in several CNS pathologies and it’s used in neurological models of these pathologies; LCS has been prepared synthetically and used as modulator of lipids metabolism in biological research, indeed it is a potent inhibitor of SPT activity. Furthermore, it has been demonstrate that LCS inhibits mouse brain SPT activity in vivo after intraperitoneal injection. [50]
Figure 15. “Conformations” and “ structures” of D- and L- α-amino acids inhibitors of SPT. [49]
LCS and DCS react with PLP forming an external aldimine that undergoes to decyclization of the isoxazolidone ring. A 15-fold higher concentration of DCS is needed to obtain SPT inhibiyion in the same extent and over the same time in comparison with LCS showing a clear enantiospecific difference of
Introduction
23 the enzyme active site. Furthermore incubation of treated samples in a buffer containing 25 M PLP caused the recovery of SPT activity whereas dialysis in buffer (without PLP) didn’t cause any further change of activity (the enzyme is still inactivated) confirming the disabling of PLP activity and the absence of further covalent modifications.
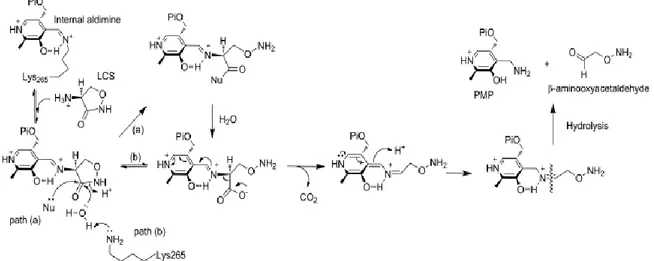
UV-spectra changes of the PLP-cycloserine aldimine adduct over time suggested the formation of new different species. A decarboxylative ring-opening mechanism for inactivation of SPT has been demonstrated: reaction intermediates (PMP and β-aminooxyacetaldehyde) have been identified by LC-ESI-MS analyses of SPT samples incubated with LCS. Successively two different decarboxilative pathways can take place: a) the ring-opened intermediate is acylated (enzyme mediated mechanism) and hydrolysed to form the carboxylated PLP intermediate (path a Figure 16); b) ring-opened adduct could proceeds through acid catalysis with water nucleophilic attack on the CS ring (path b Figure 16). [49]
Figure 16. Novel ring-opening, decarboxylative mechanism for inactivation of SPT by LCS. Path (a) denotes an enzymatic, nucleophile (Nu)- mediated mechanism with an acylated intermediate. Path (b) is the direct
hydrolytic mechanism. [49]
Myriocin
Myriocin [(2S, 3R, 4R, 6E)-2-amino-3,4-dihydroxy-2-(hydroxymethyl)-14oxo-6-eicosenoic acid] (Figure 17) also known as thermozymocidin and ISP-1, has potent immunosuppressant properties in addition to antibacterial and antifungal activity.
Serine palmitoyltransferase
24
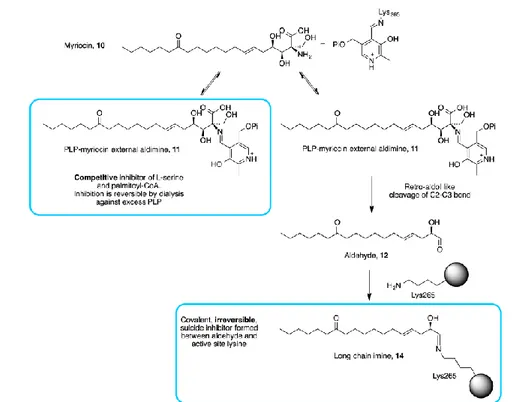
Firstly isolated by thermophilic Myriococcum albomyces and Mycelia sterilia, myriocin remains the most valuable and widely used chemical probes in sphingolipids research as demonstrated by several studies. Despite its use, the molecular basis of myriocin inhibition of SPT is largely unknown. Campopiano and coworkers have recently shed light in molecular mechanism of SPT inhibition by myriocin. UV-Vis analysis after addition of five-fold molar excess of myriocin to holo-SPT revealed transamination reaction between PLP and myriocin leading to the formation of a stable external aldimine mimicking the β-keto acid intermediate which was confirmed by LC ESI-MS analyses.
SPT:PLP-myriocin complex has a noncovalent, reversible nature and very slow off rate (koff) when incubated with myriocin for 10 minutes. Moreover,
experiments demonstrate that myriocin is a competitive inhibitor for both L-serine and palmitoyl-CoA (Ki of 967±98 nM). On the other hand a covalent and irreversible nature of myriocin-enzyme interaction has been observed when samples were incubated for 16 hours. Mass spectrometry analyses showed a covalent adduct of SPT displaying a mass of 47.509 Da susceptible to NaBH4. Peptide mass fingerprinting identified Lys
265
as the site of modification. Trypsin digest and mass spectrometry analysis identify three peptide species with monoisotopic masses corresponding to Lys265 modified by Δ mass +282.24. On the basis of this data a retro-aldol like mechanism of SPT-PLP-myriocin complex which selectively and covalently modifies the Lys265 (crucial for the enzyme-catalyzed reaction) was hypothesized (Figure 18). The PLP-myriocin external aldimine (11 Figure 18) degrades into the corresponding C18 aldehyde (12 Figure 18). This mechanism requires a base to remove the proton from the 3-hydroxy group of myriocin but Lys265 would be in wrong face to perform this role. However, the absolutely conserved His159 is positioned 2.6 A° away from the 3-hydroxy of myriocin and probably initiates the cleavage of the C2-C3 bond with the electrons sinking into the PLP ring. In the other hand deprotonated Lys265 is positioned to attack the newly formed C18 aldehyde species 12 to form a covalent aldimine adduct (14 Figure 18): irreversible modification of the key catalytic lysine residue prevents regeneration by PLP on a biologically-relevant timescale. [51]
Introduction
25
Figure 18. Myriocin reacts with PLP in the active site to form the inhibitory PLP-myriocin aldimine 11, this species is stable for greater than an hour at physiological temperature with inhibition being reversible upon
addition of excess PLP. [51]
3.6 Targeting SPT
Retinitis Pigmentosa
Retinitis pigmentosa (RP) is a general term related to a wide range of rod and cone dystrophies characterized by progressive night blindness, visual field constriction and loss of acuity. Currently, there is no therapy able to stop the disease’s evolution.
A useful model for human RP is represented by Rd10 mice line which mimics a form of human autosomal RP. Increased Rd10 mice retinal ceramide levels has been detected at the third week of life, which correspond to the maximum photoreceptor death period as well as in the human RP. These high levels were constant for the following period, whereas in wild-type mice ceramide levels decreased during the same period reaching a plateau after full retinal maturity. Rod degeneration presents the characteristic features of apoptosis. In Rd10 mice model of RP, single intraocular injections of 0.5 nmol of myriocin, significantly decreased ceramide levels (17.5%) than in control, and rescued photoreceptors from apoptotic death whereas the ceramide levels in wild type were not significantly changed. Non-invasive and long term treatment with eye drops (consisting in a suspension of solid lipid nanoparticles loaded with myriocin) ameliorated function loss and of ceramide levels (40.6%). Histological analysis demonstrated that myriocin treatment increased photoreceptor survival, preserving photoreceptor
Serine palmitoyltransferase
26
morphology and extended the ability of retina to respond to light suggesting SPT inhibitors as useful tool for the treatment of RP. [52]
Metabolic syndrome
Metabolic syndrome is a disorder of energy utilization and storage caused by medical conditions such as abdominal (central) obesity, elevated blood pressure, elevated fasting plasma glucose, high serum triglycerides, and low high-density cholesterol (HDL) levels. The metabolic syndrome increases the risk of developing cardiovascular diseases, particularly heart failure, and diabetes. [53] “Cardiac lipotoxicity” is a hearth contractile dysfunction that can be induced by elevated intra-myocardial ceramide levels. A recent research [54] and a patent [55] have highlighted the important role of SPT inhibition as potential target in the treatment of myocardial insulin resistance.
Alzheimer
Elevated levels of ceramides has been detected in brain of patient affected by Alzheimer's disease (AD) and other neurodegenerative diseases. [56] Moreover high levels of SPT has been detected in AD patients brains and its inhibition caused a reduction of amyloid-β (Aβ) whereas administration of ceramide enhance Aβ levels. [57] Recently it has been suggested that increased levels of de novo synthesized ceramide causes release of pro-inflammatory factors such as TNF- and interlukin-1β which upregulate SMase. This phenomenon upregulate β-site APP-cleaveging enzyme 1 (BACE 1) through SMase pathway (Figure 19). [58] Data obtained in AD mouse models treated with the potent SPT inhibitor L-cycloserine highlighted a reduction of ceramide levels and Aβ confirming the role of SPT (and ceramide) in AD. In particular an indirect inhibition of γ-secretase by SPT inhibition has been postulated as mechanism of Aβ reduction. [59]
Figure 19. Proposed cellular mechanism by which palmitic acid (PA) metabolism induces amyloidogenesis in primary neurons mediated by astrocytes. The astrocytes in the brain metabolize PA to generate ceramides through the de novo ceramide synthesis pathway by SPT, which initiates the release of soluble molecules, i.e., tumor
necrosis factor (TNF)-α and interleukin (IL)-1β, from the astrocytes. In turn, these soluble molecules activate the sphingomyelinase (SMase)-ceramide pathway in the neurons to increase ceramide levels. Upregulated ceramide increase
β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) level and enhance amyloid-β (Aβ) production. The increased extracellular Aβ level may act on both the astrocytes and the neurons to enhance the intracellular ceramide
Introduction
27 Hereditary Sensory Neuropathy Type 1 (HSAN 1)
HSAN 1 is a neuropathy characterized by loss of pain, temperature sensation in hands and feet accompanied with skin ulcers, infections and high pain. In addition, degeneration of motor neuron occurs with consequent atrophy and weakness of distal muscles of hands and legs. Today it has been identified missense mutation at gene encoding the first subunits of SPT. Four missense mutations associated with HSAN 1 have been reported corresponding to C133W, C133Y, V144D, and G387A. The most frequent mutation observed is an C133W, while G387A is a relatively not common mutation and doesn’t look like disease-causing mutation. The total sphingolipids levels are not increased in SPTLC133W transgenic mice that develop an age-dependent peripheral neuropathy with motor and sensory impairments. Cells can also generate ceramide by the degradation of sphingomyelin from external sources and therefore they are principally able to compensate reduced de novo ceramide synthesis. [60, 61]
Experiments in the HEK293 cell lines expressing the SPTLC1 mutants C133W and C133Y (HEKC133W, HEKC133Y) revealed the presence unusual metabolites characterized by sphingoid backbone with lack of the hydroxyl group at the C1 therefore called deoxy-sphingoid bases (DSBs). The two identified metabolites originate from the conjugation of palmitoyl-CoA with alanine and glycine instead of serine. This reaction would result in the formation of the two atypical sphingolipids with the lack of the hydroxyl and hydroxymethyl groups at C1 (Figure 20).
Figure 20. (A) Products of the SPT reaction using serine, alanine, or glycine as substrates. The conjugation of palmitoyl-CoA with alanine and glycine leads to the formation of the two DSBs: m18:0 and m17:0. (B) Chemical structure of the DSBs. The numbers of hydroxyls are designated by m (for mono-) and d (for di-)
followed by the number of carbons. The second number indicates the double bonds. For example, d18:0 stands for sphinganine, and d18:1 stands for sphingosine. All shown metabolites were also found in the
Serine palmitoyltransferase
28
Indeed, when HEK133W and HEK133Y cells are treated with alanine (10 mM) and glycine (10 mM), and the de novo synthesis is blocked with fumosin B1, the levels of 1-deoxy-sphinganine and 1-deoxymethyl-sphinganine were increased, respectively, 4-fold and 10-fold suggesting that the HSAN1 mutations induce a shift in the substrate affinity of SPT from L-serine toward alanine and glycine. DSBs are successively metabolized but not in the classical pathway. The lack of the hydroxyl and hydroxymethyl groups inhibits the formation of higher substituted sphingolipids, and their degradation by the classical pathway because of the inability to form a phosphoester bond at C1. Therefore, DSBs are substrates for ceramide synthase, and ceramide desaturase (DES), forming deoxy-ceramide and methyl-ceramide (demonstrated by the elevated levels of deoxy-sfingosine and deoxy-methyl-sphingosine in lipid extraction). In the human HSAN 1 plasma higher levels of unsaturated DSBs (than saturated type) has been detected. Furthermore, the highest DSBs levels are related with the most severe HSAN1 phenotype. Immune-fluorescence analyses suggested that DSBs change the stability and dynamics of neurofilament formation: the neurofilament straining is significantly shortened and only partly co-localized with the actin. The actin is detected over the whole length of the neuritis suggesting that the pathological mechanism in HSAN1 is the accumulation of these neurotoxic metabolites rather than the reduced de novo sphingolipid synthesis. [61]
High levels of DSBs has been detected also in diabetic neuropathy and might be responsible for other neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS). [62]
Aim of the Thesis
31
Aim of the Thesis
Apoptosis is programmed cell death and this events represent an important biological target in development of anticancer and anti-neurodegenerative drugs. The main effector of this biochemical event is ceramide. Increased levels of this sphingolipid enhances apoptotic cell death allowing to reduce tumor growth. On contrary decreased ceramide levels reduce apoptotic events avoiding uncontrolled neurons death (anti-neurodegenerative effect).
Ceramide levels can be modulated directly interfering on biological pathway responsible of its production and degradation or indirectly having effects on others endogenous system which are able to stimulate/inhibit ceramide mediated apoptosis.
During my PhD both strategies has been pursued for the development of anticancer and anti-neurodegenerative drugs targeting the de novo synthetic pathway of ceramide and targeting the endocannabinoid system.
A direct modulation of ceramide levels might be obtained by inhibition of an pivotal enzyme involved in the de novo ceramide pathway: serine palmitoyltransferase (SPT). Reduction of ceramide levels by SPT inhibition might represents a new therapeutic approach against neurodegenerative diseases caused by elevated levels of ceramide.
Indirect modulation of ceramide levels (through activation of TNF or de
novo biosynthetic pathways) by endocannabinoids receptor activation or
MAGL inhibition represent a powerful tool in struggle against cancer. Moreover selective stimulation of CB2 receptor leads to antinflammatory
effects which reduce neurodegenerative events.
As previously defined my PhD research has been focused on targeting the endocannabinoid system (Section 1) and the de novo synthetic pathway of
Aim of the Thesis
32
ceramide (Section2). In particular different goals has been pursued and reported in this thesis:
SECTION 1. Targeting the endocannabinoid system
Design and synthesis of [18F]-labelled nitrogen heterocyclic derivatives as new candidate for cannabinoid CB2 receptor PET imaging
Assessment of a high-performance liquid chromatography assay for
human recombinant monoacylglycerol lipase activity SECTION 2. Targeting the de novo ceramide synthesis pathway
Design, synthesis and biological evaluation of new SPT inhibitors Development and optimization of an HPLC-FL method to screen
SPT inhibitors
Evaluation and characterization of bacterial SPT inhibitors An insight into bacterial SPT
![Figure 5: Biodistribution preliminary data of [ 18 F]C](https://thumb-eu.123doks.com/thumbv2/123dokorg/8014775.121696/53.893.204.710.870.1086/figure-biodistribution-preliminary-data-f-c.webp)
![Figure 6: Superimposed UV profiles of LC-MS for the radiolabelling of 7 for reaction mixture, [H]-A standard and A standard (left)](https://thumb-eu.123doks.com/thumbv2/123dokorg/8014775.121696/54.893.146.673.415.615/figure-superimposed-profiles-radiolabelling-reaction-mixture-standard-standard.webp)