Chapter 2
Literature Review
2.1 Introduction
In this chapter a brief outlook on the literature about the raw materials used in the present work (polypropylene, nano-clay and PPgMA) will be presented. A concise ‘history’ of the recently ‘born’ polymer/organo-clay NCPs will be discussed. Their properties will be described and outlines will be given of those ones not properly matter of this thesis. The last part of the chapter will be dedicated to the examination of the thermodynamic features of NCP preparation.
2.2 Polypropylene
2.2.1 General features
A fast growth of PP production, in comparison to other thermoplastics, has been encouraged by significant advances in catalyst and process development and by the attractive combination of features which characterizes this polymer such as low density, heat distortion temperature above 100°C, extraordinary versatility in terms of properties, applications, and recycling in addition to a low cost1.
PP is a vinyl polymer obtained, by means of organo-metallic catalysts2, from propylene (CH2-CH-CH3) through the reaction:
Depending on the organization of CH3 groups along the backbone, three configurations can be distinguished: isotactic, syndiotactic and atactic. When all the groups are on the same side of the chain, PP is isotactic: in this case chains have a very regular arrangement; consequently they can pack easily together in
a crystal structure. Thus, the higher the amount of isotactic phases in the polymer, the higher the degree of crystallinity in the solidified PP. In syndiotactic configuration, methyl groups are alternatively on either side of the carbon chain. This structure yields a highly flexible PP. No order at all is present in atactic PP: the side groups are randomly situated on either side of the backbone and therefore the polymer has a very low degree of crystallinity. Compared to isotactic PP, the syndiotactic isomer ‘exhibits higher impact resistance and improved adhesion on organic surfaces or glass fillers’3.
However, PP is semi-crystalline, which means it always has two phases, an amorphous and a crystalline one. When cooling is slow enough, molecular chains have time to arrange themselves in ‘lamellar’ fibrils. These structures grow out from a central nucleus in the three dimensions yielding an organization with a spherical symmetry. The whole assembly is called ‘spherulite’ and is illustrated in Figure 2.1.
Figure 2.1: Schematic illustration of chains organization in PP spherulites2
Thus it is possible to distinguish: spherulites on the largest scale, the lamellar structure on the intermediate level and the crystal structure on the smallest scale2.
PP is a polymorphic material. This means its crystals can be arranged in several forms: monoclinic a, hexagonal ?, triclinic ? or smectic ?. The formation of a certain crystal form will depend on the crystallization conditions4.
However PP is usually produced and commercialised as a copolymer: in this case different monomers are used as raw materials. Ethylene is the monomer most commonly copolymerized with propylene. Copolymerization level and kind (random, block, alternate) strongly affect the final material properties: for instance if 5% of ethylene is random copolymerized, the crystallinity degree is already markedly reduced2.
2.2.2 PP properties
‘Polypropylene’ is a very versatile polymer, thus an exhaustive discussion of all its features would be too vast and inappropriate in the present work. Moreover PP is a well known material, hence, in this paragraph, only the properties which might be useful for a deeper comprehension of the subject of this thesis will be briefly outlined.
The PP mechanical properties, as stiffness and ductility, are strongly affected by its crystallinity degree. The latter, though, does not depend only on tacticity but it is influenced by thermal history as well: for instance, quenching the polypropylene, the biggest part of polymer will be ‘frozen' in an amorphous arrangement, more the few crystals formed will be small. Such a material will be less stiff than the slowly cooled one. Consequently several variables could be moved in order to ‘set’ the final material properties according to our need. For this reason it is necessary, in listing PP general features, either to state what grade of material we are dealing with or to refer to ‘average’ values.
For a 100% isotactic PP the significant numbers are5,6: - Melting temperature: 174°C
- Glass transition temperature: -17°C - Amorphous density at 25°C: 0.85 g/cm3 - Crystalline density at 25°C: 0.95 g/cm3 - Average tensile yield strength: 30.7 MPa - Average Young’s modulus: 1.9 GPa - Service temperature up to 120°C
In general, PP can be considered chemically ‘inert’: namely its very stable structure provides a good chemical resistance, except for aromatic hydrocarbons at elevated temperatures and halogens. It can even withstand most inorganic acids, except nitric and sulphuric, and inorganic salt solutions. On the other hand PP is unsuitable, due to swelling, to be used with most organic solvents6. These features, together with water resistance, low density and good price/property ratio, give to PP its great industrial importance4.
As an example Table 2.1 gives the mechanical properties and relative cost of some plastics.
Table 2.1 Principal properties and relative cost of commercial polymers from ref. 6
Material Tensile strength (MPa) Elastic modulus (GPa) Density (kg/m 3 ) Relative cost Polypropylene 35 1.5 900 1.0 PVC 55 3.5 1400 1.0 Polyethylene 12 0.2 900 0.7 PTFE 21 1.0 2100 20.0 GRP polyester 100 7.0 1500 2.0 GRP epoxy 250 14.0 1800 3.3
However, as already said in paragraph 2.2.1, PP is more commonly commercialised as copolymer and/or loaded with fillers: in fact its brittleness at low temperature and its low resistance to oxidation with light, heat and oxidants
restricts the use of the neat polymer. Nonetheless, Table 2.1 shows PP big advantage: it is a cheap polymer; therefore, major efforts have been dedicated to improve its properties instead of looking for a different polymer. That is why PP is currently toughened through the already mentioned ethylene copolymerization and the problems coming from its low stiffness, low heat distortion and its high mould shrinkage are overcome by incorporating glass and mineral fillers (such as talc at a micro scale)7,4,8. In Table 2.1 it is also evident the reason why the companies sponsoring MACRO project are looking for an appropriate substitution for PTFE as antennas substrate: its cost is about 20 times that of pure PP. All this information is definitely not enough to have an exhaustive overview of PP potentiality, but it helps to appreciate its complexity and, at the same time, its flexibility which makes of it one of the most interesting thermoplastic to spend time, efforts and research on.
2.3 Clays
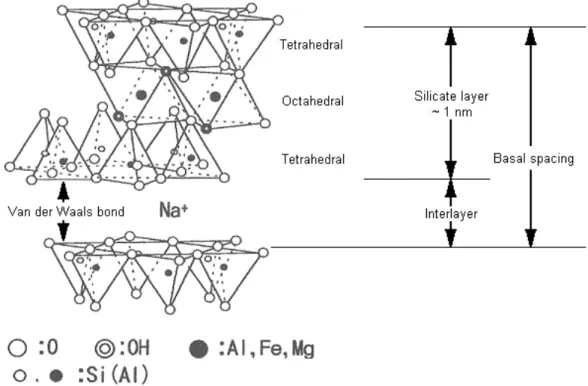
Constitution of common clays is subject to natural variability since they are naturally occurring minerals; besides their pureness can affect the final NCP properties. However, many varieties of clay are aluminosilicates with a layered structure which consists of silica (SiO44-) tetrahedral sheets bonded to alumina (AlO69-) octahedral ones. These sheets can be arranged in a variety of ways; in smectite clays a 2:1 ratio of the tetrahedral to the octahedral is observed.
Montmorillonite (MMT) is the most common of smectite clays9.
As clearly shown in Figure 2.2, in MMT, oxygen atoms from each alumina octahedral sheet also belong to the silica tetrahedral contiguous ones, the three of them consisting of 1 nm thin layer. These layers are in turn linked together by Van der Waals bonds and organized in stacks with a regular gap between them called ‘interlayer’ or ‘gallery’.
Within the layers, isomorphic substitution of atoms as Al3+ with Mg2+ or Fe2+ can occur generating an excess of negative charge, the amount of which characterizes each clay type and is defined through the charge exchange
capacity (CEC). The CEC value for MMT depends on its mineral origin, yet is
typically 0.9-1.2 meq/g. In natural clays ions such as Na+, Li+ or Ca2+ in their hydrated form balance this excess negative charge; this means natural MMT is only miscible with hydrophilic polymers like polyethylene oxide and polyvinyl alcohol11,12,13.
In order to make possible for MMT to be miscible with other kinds of polymers, it is essential to replace the alkali ions with cationic-organic surfactants, such as alkylammonium ion14,15. This is the surface treatment mentioned in the Introduction. In this way the natural clay can be rendered ‘organophilic’ and then named ‘organo-clay’, or ‘clay’, that is to say capable of yielding a nano-composite interacting with an organic matrix. For further details concerning the surface treating agents used in this study, see paragraph 3.2.2. Several other surface treatments can be applied to the clays in order to make them organophilic and more likely to disperse in the polymer matrix as explained in paragraph 2.4.2.
It is worth saying that the layers (platelets) have been proven to be not totally rigid, but have a certain degree of flexibility9, and this feature can affect the mechanical behaviour of the resulting composite.
2.4 Organo-clays and Nanocomposites
2.4.1 General features
Due to the very recent nanocomposite birth, looking for this word in a chemical dictionary is not even possible to find it. All one can find is ‘composite’ (‘A mixture or mechanical combination on a macroscale of two or more materials that are solid in the finished state, are naturally insoluble, and differ in chemical nature’16) and ‘nano-’ (‘A prefix representing 10-9, which is one-billionth of the unit adjoined’17).
However it is perfectly known that polymer/clay NCPs represent ‘a new class of plastics derived from the incorporation of nanoscale particles into polymers’18. In recent years they have received increased attention, both in industry and in academia, by virtue of their enhanced mechanical and physical properties. A large amount of work has been done concerning them, using polyimide19, epoxy resin20, polystyrene21, polycaprolactone22, acrylic polymer23, polybutadiene24 and polypropylene25,32,26.
Although different types of clay can be used for nanocomposite formation, depending on the various properties demanded to the product, MMT is the most common one.
However, mica and hectorite have been studied as well in comparison with MMT and the results showed that, albeit MMT and mica resulted in a poorer dispersion of the layers in the polymer matrix than hectorite, the properties of their resulting NCPs had higher improvements. This behaviour probably depends on the fact that MMT and mica platelets seem to be stiffer than hectorite ones, suggesting a fundamental idea in NCPs studying: sometimes higher reinforcement effects can be achieved regardless of the worse separation of the stacked structures27. This idea has been supported by Svoboda et al. as well: according to them a better dispersion does not mean necessarily improved mechanical properties. On the contrary the co-existence of both well dispersed clay and large clay tactoids* can be a positive aspect: in fact the tactoids act as a nucleating agent in PP decreasing the size and increasing the number of spherulites in a given volume and, as a consequence, increasing impact strength1.
Synthetic clays (e.g. hydrotalcite) have also been used because they can be produced in a very pure form and can carry a positive charge within the layers, instead of the negative one found in MMT9.
As already said, platelet thickness is of the order of 1 nm, whereas their aspect ratios can be very high, typically 100-1500 and it has been already demonstrated that a clay mineral filler with a larger aspect ratio (in combination with adequate interfacial adhesion between filler and polymer28) can be responsible of significantly improved reinforcement effects on the final composite29.
Accordingly, as discussed in the work of Svoboda et al., ‘the addition of clay to PP always improves the tensile strength and tensile modulus, but reduces its ultimate elongation’. ‘The most significant increase in tensile strength occurs with the addition of 1 to 2% of clay. Further addition of clay mainly improves the tensile modulus.’ ‘For a clay content superior to 7%, the material does not show any yielding behaviour, it breaks after having reached its maximum tensile stress’1. These percentages show another positive aspect of working with nanofillers: the chance of keeping the loading level at low values. In fact, due to their nano-scale dimension, a single gram of clays will contain over a million individual elements. This means that, once the particles are exfoliated, a high concentration of units will be reached anyway, even though a very low amount was added.
In order to look for an optimum in NC loading, another aspect has to be taken into account: two studies1,30 concur in reporting that, when a high molecular weight PPgMA is used as compatibilizer, the clay spacing increases greatly as the clay content decreases.
Finally it is important to highlight that the NC particles are resistant to solvents, to polymerization temperatures and to compounding shear. As a result, they can be processed without concern about degradation. This fact has two fundamental consequences: firstly the NCP can be formed and treated in the same devices
*
used for the neat polymer and, secondly, no restrictions will be given by the filler to the NCP recycling31.
2.4.2 Surface treatment
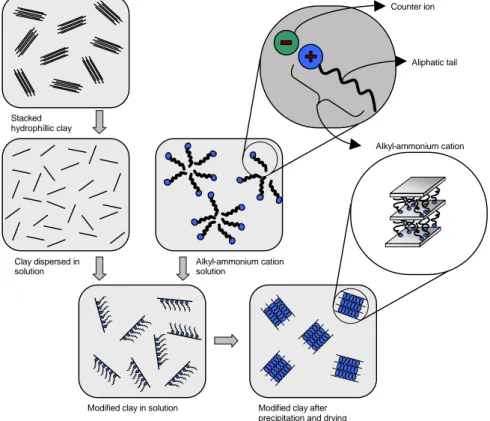
The modification of clay polarity to provide the ‘organophilic’ character to the clay is an essential requirement for successful formation of polymer-clay NCPs. Organophilic clay can be formed from normally hydrophilic clay by means of an ion exchange with an organic cation such as the already mentioned alkylammonium ion.
Not only the chemical product used as treating agent, but the way in which this substitution is performed has an effect on the formation of particular nanocomposite product forms. However, the laboratory route commonly used to introduce alkylammonium ions in the interlayer is an ion exchange reaction which consists of promoting the formation, in solution, of the desired ion dissolving either the related amine together with a strong acid32 or a salt which has long alkyl chain cation linked to counter-ions as chloride or bromide33 (schematically illustrated in Figure 2.3) into hot water (about 80°C). Such solution has to be poured into MMT previously dispersed into hot water as well. A vigorous stirring with a homogenizer is required to yield white precipitates which have to be collected, washed and eventually dried.
Figure 2.3: Schematic representation of clay surface treatment
Obviously this treatment adds to the cost of the clay but, on the other hand, the latter are relatively cheap as raw materials.
Stacked hydrophillic clay Clay dispersed in solution Counter ion Aliphatic tail Alkyl-ammonium cation Alkyl-ammonium cation solution
Modified clay in solution Modified clay after precipitation and drying
It is important to note the surface treatment task is not only to render the clay an organo-clay, improving the wetting characteristic with the apolar polymer, but it is to increase the interlayer distance as well. Indeed surface treated clay is used even in case of polar polymers in which the modification of clay polarity is not fundamental for the NCP production.
Clearly, as the amount of carbon atoms in the tail of the ammonium ion increases, the clay becomes more organophilic; more, the introduction of a longer organic molecule in the clay structure helps to increase the interlayer distance as well. For this reason hexadecyl-trimethyl-ammonium ion33 or dioctadecyl-dimethyl-ammonium ion34 can be used.
Some experimentation has been done trying to improve the surface treatment efficiency because silicate layers, even modified by apolar long alkyl groups, are still polar and thermodynamically incompatible with polyolefin (see paragraph 2.5). An alternative way to the ordinary organophilic clay has been suggested by Liu et al. and it consist of co-intercalating in clay stacks an unsaturated monomer which promotes both a larger interlayer spacing and the possibility for the monomer to tether on the PP backbone by a grafting reaction33.
Another option in terms of surface treatment has been given by Manias et al., nevertheless their work needs to be discussed after a thermodynamic background about the topic has been given, so it will be examined at paragraph 2.5.
2.4.3 NCP properties
In general, using NC as filler in thermoplastic polymer matrices has many points of interest. In particular, when polypropylene is involved, the main attractive aspect, discussed in many articles, is a significant increase in its stiffness35,32,36. However, using clay in a PP matrix leads to many other largely reported improvements, such as:
- increased tensile strength and modulus (probably because the layers contribute to partially immobilize a certain amount of polymer phase); - increased dynamic stiffness;
- accelerated crystallisation (almost certainly because the clays act as nucleating agents);
- not affected crystal structure of the PP matrix33; - improved flame retardancy37;
- enhanced barrier to gas (oxygen and carbon dioxide), water and hydrocarbons (gasoline, methanol, and organic solvents) permeation; - lower density;
- better scratch resistance even for very modest NC loadings (1-5%)38; - increased heat deflection temperature35.
Even though it has been demonstrated that not all of these properties depend on dispersion level (such as the flame retardancy37), Hasegawa et al. revealed remarkable enhancements of the clay reinforcement effect (mainly in the mechanical aspects) by improving the inorganic silicate layers dispersion32. This behaviour, in all probability, depends on the fact that enhancing the dispersion level, the particles act as individual units increasing the specific
surface area of the filler which can even reach 700 m2/g39. Such a large interfacial area per volume leads to a high fraction of interphase (i.e. interfacial material) on which the final composite properties strongly depend.
Loadings of 2-10 wt% of nano-filler can yield property enhancements equal to those expected from a conventional composite containing 20-35% mineral or glass31. Hence it is correct enough to say the key word in NCP studies is ‘dispersion’: it represents the level to which the clay particles are dispersed within the polymer matrix. Usually the aim of the NCP manufacturer is to realize a state in which is no more possible to distinguish any polymer or filler rich areas in the final material. When this happens, the X-ray diffraction pattern shows no peak and this means there is no longer an ‘order’ within the clay particles; instead of agglomerated arrangement they appear as individual platelets within the polymer: they are exfoliated. In order to achieve this goal a limit value in NC loading has to be respected: above the latter an inevitable aggregation of the clay occurs and the dispersion (with all the properties depending on it) is no more increased1.
On the other hand, as already said in paragraph 2.4.1, special emphasis is lately being placed upon the idea that an appreciable improvement of mechanical properties does not necessarily depend on the complete NC exfoliation, although a homogeneous distribution is still a fundamental pre-requisite for successful NCP formation.
Another aspect which raises even more the interest in NCPs is the fact that rheological studies have demonstrated that the NCP flow characteristics are nearly the same as the neat PP when the NC loading level does not exceed 6%. This result leads to believe this new material can be processed in the current machine set up, without adding extra cost to the producer and, as a consequence, to the end users35.
Beyond the aforecited improvements, NCPs can claim parallel enhancements of other material properties which, in turn, may represent the major promise for any potential future uses of PP/MMT NCPs, given that tensile properties can also be improved by other means40,41. In this paragraph a brief outlook on these apparently secondary aspects will be given.
MMT layers are just 1 nm thin and, even though their lateral size can be of the order of micrometer, the NCP results to be optically clear in the visible region, at least when the platelets are well dispersed in the matrix. For NC loadings up to 9% no noticeable decrease in UV transmittance and in the clarity is observed. Roughly 20% of NC is necessary before a perceptible loss in transparency in 3 mm thick films of PPgMA can be detected. Besides, this loss of intensity in the UV region seems to depend mostly on the presence of tactoids with no scattering contributes from the exfoliated layers. Thus, a fully exfoliated NCP should, very likely, appear as optically clear as the pure polymer11.
Other properties depend on the sheet-like structure of the filler: in fact, the nanometre-thin layers act as obstacles to molecules diffusing towards the NCP bulk, extending their path through the polymer and, thus, decreasing its permeability to gases and liquids. The latter is, as a matter of fact, reduced to
half when only 4% of MMT is dispersed in the poliolefinic matrix, and the solvent uptake is accordingly reduced38. Obviously an appreciable level of platelet orientation is required to provide a significant gas barrier enhancement as illustrated in Figure 2.4.
Figure 2.4: Nano-clay improves gas barrier properties
The flame retardancy of PP, together with a reduced smoke emission, is also promoted by NC. This enhancement is quantitatively represented in Figure 2.5, where the mass loss rate (evaluated by cone calorimetry) is plotted versus time of combustion. The patterns clearly show a sharp reduction in flammability already with 2% of NC; reduction which reach the 75% (compared to the unfilled polymer) when the loading is 4%42.
Figure 2.5 from ref. 42 where there also exists a detailed discussion of the general mechanism responsible for the improvement in flammability in clay reinforced polymers
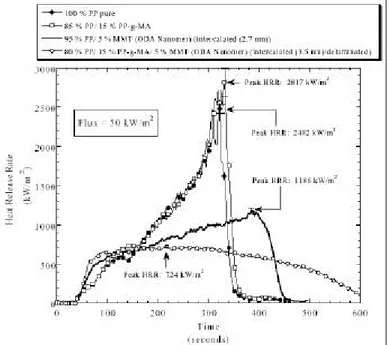
Figure 2.6, in addition, shows the compatibilizer effect on flame retardancy. In this case the heat release rate is plotted versus time and it is evident that even keeping the NC loading constant, the introduction of 15% of compatibilizer (and
Unfilled polymer Tortuous path in NCP
Tortuous path in oriented NCP
hence the increasing on interlayer distance from 2.7 nm to 3.5 nm) contributes to lower and broaden the curve.
Figure 2.6 from ref. 37
On the other hand, Figure 2.7 illustrates that there is no need to exceed the loading of 5% since the use of 10% of NC yields only a moderate benefit.
This behaviour seems to depend on the fact that the nano-filler allows the formation of a carbonaceous-char layer on the outer surface during combustion. This ‘skin’ acts as a heat and mass transport blockage insulating the polymer bulk and delaying both the oxygen supply and the release of the combustion products.
Figure 2.8 from ref. 37
Figure 2.8 depicts the images of the crucibles used in the gasification experiments, the composition being: PP with 5% of NC for the left sample, PP with 2% of NC and 15% of compatibilizer for the centre sample and PP with 5% of NC and still 15% of compatibilizer for the right sample. Comparing the first two pictures results clear the importance of using both a nano-filler and a compatibilizer to form a carbonaceous char layer whereas the last two pictures show how a higher NC loading can improve its volume and quality.
However it is worth noting that, despite the fact NCPs show a reduced flammability, they have a sort of shorter time of ignition probably depending both on physical effects (such as higher thermal conductivity and radiation absorption) and on chemical ones (such as volatile organic treatment)37.
Moreover, since only a few percent of NC are needed in NCPs formation, they do not add noticeable weight to the resulting composites.
Furthermore NC can be incorporated in the final steps of already existing polymer production lines (e.g. extrusion, injection/compression moulding) to obtain NCP hybrids thus promoting their future commercialization.
At last, NC fillers can also be used together with other common PP reinforcements, such as fibres, combining, in such way, the PP/NC unique improvements and the traditional ones from the conventional reinforcements in one composite-nanocomposite material38, which is exactly the idea that promoted this thesis.
2.4.4 Routes leading to PP-NC nanocomposite
Filling polymers with clays (both synthetic and natural with appropriate modification) is not a completely new subject43,44, nonetheless two findings initiated the revitalization of these materials in the last decade.
The first one is the report in 1993 of a MMT/nylon 6 NCP from Toyota Group research45,46. The obtained NCP contained separated MMT layers homogeneously dispersed throughout the nylon matrix. Significant enhancements of both thermal and mechanical properties were observed in spite
of a very moderate inorganic loading. Notwithstanding this NCP did not gain applicative success owing to a very long preparation method which increased too much the final material cost.
The second work boosting the subject was from Giannelis et al. who found a procedure leading to NCP by melt mixing polymers with clays (intercalated with organic cations) without using organic solvents21.38 Unfortunately this technique was fruitfully applicable only to polymers with polar groups in their backbone and not to non-polar ones such as polyolefin in general and PP in particular27.
Ever since, a lot of new techniques were tested in order to find a feasible route leading to NCPs without increasing too much the process complexity and, consequently, the cost. However it is possible to distinguish four distinct strategies which can be used to prepare NCPs.
2.4.4.1 Solvent route
According to the first one the polymer is initially solubilized in an organic solvent, then the clay is dispersed in the obtained solution and subsequently either the solvent is evaporated or the polymer precipitated47,48,49.
Nevertheless this technique leads to poor clay dispersion, besides it is applicable only to polymers which are soluble into common solvents. More, since a large amount of solvent needs to be used to achieve appreciable filler dispersion, and the same quantity of solvent has to be removed after mixing, this method results to be long and tedious, then suitable only for laboratory applications.
2.4.4.2 In situ polymerization
In the second strategy the problems presented by using a solution medium have been overcame by mixing the silicate layers with the monomer46,22,50, in conjunction with the polymerization initiator and/or the catalyst51,52,53, The following reaction is then an intercalative polymerization of the monomer started either thermally or chemically. The intercalated monomer is not necessarily the same used to obtain the matrix bulk: it could be, for instance, diacetone acrylamide27.
By this method the formation of NCP with non-polar polymers is also possible. In fact, starting from monomers, a more favourable thermodynamic for non-polar polymers/silicate miscibility occurs. Actually non-polar monomers can penetrate more easily than their respective polymers in the interlayer gallery because of a smaller entropic loss in intercalating (see paragraph 2.5).
2.4.4.3 Melt intercalation
In the third strategy, which is the one studied by Giannelis et al. and mentioned at the beginning of this paragraph, instead of passing through the monomer mixing, the silicates are directly dispersed into the melt polymer. In order for this method to be effective, though, the silicates needed to be previously surface treated through the organo-modification already discussed in 2.4.2.
The melt intercalation is more flexible and ‘green’ than the previous two routes thanks to the absence of solvent34 and chemical reaction; besides its testing with nylon-6, polysiloxane and even polystyrene yielded noteworthy results.
On the other hand, when non-polar matrices are involved54,55,21,56, a third component, such as PPgMA, has to be added to the polymer-NC system, even though the clay was already organically modified. The aim of this new costituent is to improve the matrix filler interactions32,57 by reducing the interfacial tension between them (for an exhaustive explanation of compatibilizer behaviour see paragraph 2.4.5).58
2.4.4.4 Swelling agent route
Another approach which is worth to mention is technically similar to the melt intercalation but in principle not far from the hybrid formation via solution medium. The novelty, in this case, consists of intercalating a ‘swelling agent’59 in the interlayer of the alkylammonium-exchanged MMT. This agent must have its boiling point below the material processing temperature (about 250°C) so as to evaporate when the swollen organo-modified clay is extruded together with the PP matrix. The aim is to aid the separation of platelets by means of the increase in volume which the swelling agent undergoes while evaporating. Some examples of swelling agent can be ethylene glycol, naphtha, or heptane, all with boiling point below 250°C.
When the melt intercalation approach is used, it is commonly advantageous to pass by a ‘master batch’ production: this is a sort of pre-NCP composed by a matrix of PP oligomer, already modified with either maleic anhydride (MA) or hydroxyl groups in high concentration, in which a large amount of octadecylammonium exchanged MMT (or equivalent organo-clay) is dispersed. This compatibilizer and filler rich mixture can subsequently be processed, in an extruder or mixer, with pure PP. The master batch technique major benefit is that the PPgMA pre-treated NC is at theta conditions* with the bulk PP and thus the strong mechanical shear during processing has only to promote the filler incorporation into the matrix whereas, in the melt intercalation method, the extrusion had to force the NC layers in an energetically not favourable situation.38 The master batch technique allows to reach an interlayer distance (determined with X-ray diffraction measurements) of 38 Å and an ultimate tensile test of 39 MPa (in comparison with 31 MPa of pure PP) when a NCP is prepared with 3 wt% of MMT27. This is a significant improvement coming, however, from a process which is hardly adequate to be performed out of a laboratory, even though some companies are at the moment commercialising ready-to-use master batches such as the Nanocor® C.30P used in this work (see paragraph 3.2.2.2) Cho et al. studied the influence, on its mechanical and thermal properties, of choosing either a single-screw or a twin-screw extruder to obtain a NCP by the master batch technique: the results showed that for the materials obtained by the
*
In this case theta conditions subsist between the master batch molecules and the PP matrix ones, even though these conditions are commonly referred to a polymer and its solvent as the conditions in which the polymer macromolecule has the same interactions with a solvent molecule as with monomers of polymer molecules. This definition contrasts with a ‘good solvent’ one in which interactions of solvent with the polymer are energetically favoured and the polymers expand. The theta condition will generally exist at only a single temperature (or not at all) for a given solvent. The temperature is known as the theta temperature.
two different routes both the interlayer distance and the final properties seemed to be similar, even though much better than those of the reference sample, obtained by direct compounding35.
It appears clear that, in any case, the amount of shear stress in the melt plays an important role in NCP formation. Of course it depends also on the matrix viscosity, increasing when the latter increases. However it has been demonstrated that, when PPgMA is incorporated to the mixture, a uniform dispersion can be achieved both using a low and a high viscosity PP. This means PPgMA is able to overcome the problems the insufficient shear force of the low viscosity PP matrix could generate60.
From what stated it appears evident that the processing conditions can strongly affect the properties of the resulting hybrid materials: it is possible to obtain poor dispersion and property enhancements25,57,32,27 or significant dispersion and better performing hybrids61.
Besides, NCP formation depend evidently on PPgMA functionality (namely specific content of MA, often on a weight basis) as well: if this is too low, the compatibilizer can be ineffective25, if too high, it can render the master batch too hard to be further mixed with PP57,32. 38
However more details about the compatibilizer use and efficiency will be given in the next paragraph.
2.4.5 Compatibilizer
The use of a compatibilizer, namely a chemical able to render compatible two different materials, made it possible for the melt intercalation technique to be accepted as the most promising approach leading to NCP formation. In this way the use of solvents and dedicated processes could be avoided providing a formation procedure which is both ‘environmentally’ and ‘user friendly’.
It is important, at this point, to clarify that the surface treatment and the compatibilizer are two different, independent and complementary ways adopted to solve the problem of poor miscibility between PP and clay. They act on parallel levels to overcome the same difficulty. The incompatibilities between PP and MMT are, in fact, both of thermodynamic and of physical nature. The first kind of obstacle for a successful hybrid formation is the fact that the stacks of layers in the pristine MMT form are very stable and unwilling to reach the state of disorder required to a well formed NCP (see paragraph 2.5). The second impediment to the desired exfoliated structure is the chemical unsuitability of the non-polar PP to be bonded in any way to the polar MMT platelets, at least to hold them in a non-thermodynamically favourable arrangement.
Through the surface treatment (paragraph 2.4.2) it is possible to change the interlayer structure of MMT both increasing the gallery gap and modifying the silicate surface in an organic fashion, but this artifice is not enough to render compatible matrix and filler: the ‘polarizing’ compatibilizer needs to be introduced in the PP.
In effect examples of NCPs with host polymers containing polar components such as styrene-acrylic acid copolymer already gave remarkable improvement of mechanical properties62,57.60
The polar group introduced in the hydrophobic PP backbone to make it hydrophilic is the maleic anhydride which results, from a study of Kavasumi et
al.57, to be crucial to promote the desired phase structure60. The repeating unit usually sketched to represent the resulting chain is illustrated below.
According to this representation the MA groups should be randomly grafted or block copolimerized in the PP chain. Nevertheless this kind of product is usually made by reactive extrusion with a peroxide initiator which causes a free radical formation by
scission of the PP chain. Such radical is the reactive site to which the MA group attaches. The compatibilizer vendor suggests more than one group can react with a broken PP chain leading to a dimer or even trimer formation. This means a PPgMA representation as a ‘surfactant’, where a polar head is attached to an aliphatic tail, would be more adequate than the one commonly used and depicted in the sketch above.
Hence, in simple binary mixtures of PP and organo-clay, the task of PPgMA is to establish the bond between two such different materials: the hydrocarbon part of the molecule tends to be kept in the polypropylene matrix, while the oxygen atoms in the maleic anhydride ring can be linked to the hydroxyl groups of the clays by electrostatic attraction generating a strong hydrogen bonding between them which is expected to help the exfoliation process32 (Figure 2.9 illustrates a scheme of the clay dispersion process, taken from reference 63).
Figure 2.9: Schematic representation of the clay dispersion process
In the previous paragraph has already been anticipated it exists an optimum in PPgMA functionality between a too little functionalized PP, which would be unproductive, and a functionalized PP with an excessive content of MA which, in contrast, would have too large polarity difference with the matrix PP molecules to diffuse in them60.
The PPgMA molecular weight (MW) also seems to affect some mechanical properties of the resulting NCP. Svoboda et al. tested different compatibilizer grades finding that tensile strength and impact strength actually depend on the molecular weight of PPgMA: in particular the best overall properties have been showed by the sample with the highest molecular weight (MW=330,000). On the
other side, from a dispersion point of view, such a ‘heavy’ compatibilizer hampered the diffusion of PP molecules from the bulk polymer when is used in high concentration; a higher level of dispersion is, in fact, given by lower MW PPgMA1. The same behaviour has been experienced by researchers of Crompton® (the supplier of the compatibilizer used in the present work). They reckon a plasticizer effect of the low MW molecules could be responsible for the worse mechanical properties that such NCP showed.
2.5 The thermodynamic challenge
Like everything in nature, NCP formation is thermodynamically driven and thus it can spontaneously happen only when associated with a negative free energy change (? G)60. As this principle states, dispersion, intercalation, exfoliation and, in general, hybrid formation should take place as a consequence of both entropic and enthalpic factors interaction.
According to Manias et al. ‘dispersion of MMT in a polymer requires sufficiently favourable enthalpic contributions to overcome any entropic penalties38’. In fact, the conformational entropy of the polymer chains decreases when the latter are forced to be confined inside the narrow silicate interlayer.
On the other hand, when an organically modified MMT is dispersed in a polymer, two conditions may, in theory, compensate this entropic penalty.
- The first consists of an increasing in the entropic content of the system due to an improved configurational freedom of the surfactant chains. In fact, when the layers separate, they can arrange in a less restricted environment.
- The second condition consists of a favourable enthalpic contribution obtained when the polymer and the NC are mixed. This circumstance occurs when the polymer/MMT interactions are more favourable compared to the surfactant/MMT interactions64,65,66.
This situation would be observed for the majority of systems based on polar (or polarizable) polymers and alkylammonium modified MMT.
Unfortunately, such a favourable enthalpy variation does not take place in the case of NCP based on non-functionalized PP in which the matrix molecules and the alkylammonium tails are at theta condition as they share the same aliphatic nature38. As a result, when a PP/NC mixture is concerned, the entropic aspect leads the intercalation progress.
From what stated above it should be crucial, in order to balance the penalty caused by the polymer chains confinement, to start form an initial NC structure able to yield a significant entropic increase. The latter would be theoretically be enhanced by a larger initial gallery gap and a higher organic surfactant chains packing density in the interlayer. This situation could be achieved by providing to the clay cations with longer aliphatic tails. Indeed Kim et al. found that higher initial interlayer distance and packing density is not always better: a successful intercalation would be undeniably hampered, in fact, by a too densely packed gallery which would be too tough to be further penetrated by bulk polymer chains. Accordingly, testing organo-clays with different modifiers, gallery spacings and
packing densities, the same authors confirmed this trend but, conversely, they found that the structure of the majority of them tend anyway to collapse when shear is applied during mixing. This means that, in such a binary system, the entropic gain due to the increased conformational freedom of tethered surfactant chains is not enough to endorse hybrid formation. More, the mechanical energy supplied during melt blending can lead the system to a more thermodynamically stable state.
As a result, the mixing stage, which is supposed to be an essential step in the NCP preparation to promote the kinematic factor of the process, is actually ineffective on dispersion because the matrix-filler interactions are too poor60. A similar result has been obtained by Alexandre et al. studying polyethylene/NC composites prepared by in situ polymerisation: they observed that not only the mechanical energy, but the thermal energy supply as well can be associated with a partial collapse of the exfoliated structure. All these experiences confirm what was already theoretically demonstrated64,67: an array of comparatively polar nano-layers dispersed in a non-polar matrix is thermodynamically unstable and the platelets tend to the pristine stacked structure once sufficient energy (both mechanical or thermal) is supplied to the system58.
According to Kim et al. ‘the observed instability in the binary mixture of PP and NC no doubt stems from the fact that there is no specific interaction between the two components. As a result, introduction of the third component, which can induce specific interaction, would be desirable to overcome such problems60’. Indeed in a study of Reichert et al. it is demonstrated that annealing a sample of PP/NC nanocomposite prepared by melt blending the two components with PPgMA does not lead to a collapse of the platelets. Coarsening of the structure is observed and, although such a behaviour would suggest collapsing of the layers, wide angle X-ray scattering measurements show that they become fully exfoliated under annealing68.
It is worth mentioning, in the end, an alternative approach to the ‘dispersion topic’ presented, in a recent work (2001), by Manias et al. As already said at the beginning of this chapter, the enthalpic contribution to the free energy of mixing can be enhanced designing systems in which the polymer/MMT interactions are more favourable than the surfactant/MMT interactions.
Two routes can be clearly followed in order to achieve this goal:
- Increasing the firsts through the already mentioned PP “functionalization” with MA groups.
- Decreasing the seconds.
Surely, the latter condition is more difficult to be accomplished because the interactions between the aliphatic surfactant and the MMT are already modest. In fact, when the polymer/MMT interactions are significant (namely when a polar polymer is used), these alkyl-modifiers perfectly manage to realize the aforecited thermodynamic condition.
However the mentioned authors tried the second route by introducing a semi-fluorinated surfactant as clay surface treating agent, which is expected to have less favourable interactions with the clay. Indeed such system showed
intrinsic thermodynamic stability since, starting from neat PP, a NCP was formed even unassisted by mechanical shear38.
1
Svoboda P, Zeng C, Wang H, James Lee L, Tomasko DL, Journal Of Applied Polymer Science,
85 (2002) 1562
2
School of polymers and high performance materials, The University of Southern Mississippi,
The Macrogalleria – A Cyberwonderland Of Polymer Fun, http://www.psrc.usm.edu/macrog
(accessed March 2003)
3
Stricker F, Bruch M, Mülhaupt R, Polymer, 38 (1997) 5347
4
Cozien Cazuc S, Investigation Of Polypropylene/Clay Composites, M.Sc. Thesis, Cranfield University (2002)
5
Commin L, De Blic A, Ferrari S, Silbermann V, Novel Thermoplastic Matrix Composite, M.Sc. Group Project Report, Cranfield University (2003)
6
Sinnot RK, Coulson And Richardson’s Chemical Engineering Vol. 6, Butterworth-Heinemann, Oxford, 2000, Pg. 301
7
Mills N, Plastics – Microstructure And Engineering Applications, 2nd ed. Edward Arnold, London, 1993, quoted in ref. 4
8
Waterman N, Ashby M, The Material Selector, 2nd ed. Chapman and Hall, London, 1997, quoted in ref. 4
9
Kingery WD, Bowen HK, Uhlmann DR, Introduction To Ceramics, 2nd ed. John Wiley & Sons, New York, Pg. 77
10
Kato M, Usuki A in Polymer-Clay Nanocomposites, Pinnavaia TJ, Beall GW, John Wiley & Sons, New York, 2000, Pg. 98
11
Strawhecker K, Manias E, Chemistry Of Materials, 12 (2000) 2943, quoted in ref. 38
12
Xu R, Manias E, Snyder AJ, Runt J, Macromolecules, 34 (2001) 337, quoted in ref. 38
13
Vaia RA, Vasudevan S, Krawiec W, Scanlon LG, Giannelis EP, Advanced Materials, 7 (1995) 154, quoted in ref. 38
14
Alexandre M, Dubois P, Materials Science And Engineering: R: Reports, 28 (2000) 1, quoted in ref. 38
15
Giannelis EP, Krishnamoorti RK, Manias E, Advances In Polymer Science, 138 (1998) 107, quoted in ref. 38
16
Lewis RJ Sr, Hawley’s Condensed Chemical Dictionary, Thirteenth edition, 1997, sub voce “composite”
17
Dictionary Of Scientific And Technical Terms, 3rd ed. McGraw-Hill, 1984, sub voce “nano-“
18
Medical Device & Diagnostic Industry, Enhancing Medical Device Performance With
Nanocomposite Polymers Sidebar, http://www.devicelink.com/mddi/archive/02/05/006a.html
(accessed January 2004)
19
Yano K, Usuki A, Okada A, Kurauchi T, Kamigaito O, Journal Of Polymer Science Part
A-Polymer Chemistry, 31 (1993) 2493, quoted in ref. 34
20
Wang MS, Pinnavaia TJ, Chemistry Of Materials, 6 (1994) 468, quoted in ref. 34
21
Vaia RA, Ishii H, Giannelis EP, Chemistry Of Materials, 5 (1993) 1694, quoted in ref. 58, 38, 34
22
Messersmith PB, Giannelis EP, Journal Of Polymer Science Part A-Polymer Chemistry, 33 (1995) 1047, quoted in ref. 58, 34
23
Biasci L, Aglietto M, Ruggeri G, Ciardelli F, Polymer, 35 (1994) 3296, quoted in ref. 34
24
Fukumori K, Usuki A, Sato N, Okada A, Kurauchi T, Proceedings Of The 2nd Japan International SAMPE Symposium, 1991, Pg. 89, quoted in ref. 25
25
Kato M, Usuki A, Okada A, Journal Of Applied Polymer Science, 66 (1997) 1781
26
Furuich N, Kurokawa Y, Fujita K, Oya A, Yasuda H, Kiso M, Journal Of Materials Science, 31 (1996) 4307, quoted in ref. 34
27
Oya A, Kurokawa Y, Yasuda H, Journal Of Materials Science, 35 (2000) 1045
28
Katz HS, Milewski JV, Handbook Of Fillers For Plastics, Van Nostrand Reinhold Publ., New York, 1987, quoted in ref. 68
29
Lusis J, Woodhams RT, Xanthos M, Polymer Engineering And Science. 13 (1973) 139, quoted in ref. 27
30
31
Nanocor Technical Papers, Maul PL, Plastic Nanocomposites: The Concept Goes Commercial,
http://www.nanocor.com/tech_papers/plastic_nanocomposites.asp (accessed January 2004)
32
Hasegawa N, Kawasumi M, Kato M, Usuki A, Okada A, Journal Of Applied Polymer Science,
67 (1998) 87
33
Liu X, Wu Q, Polymer, 42 (2001) 10013
34
Zhang Q, Fu Q, Jiang L, Lei Y, Polymer International, 49 (2000) 1561
35
Cho JW, Logsdon J, Omachinski S, Qian G, Lan T, Womer TW, Smith WS, Nanocomposites: A
Single Screw Mixing Study Of Nanoclay-filled Polypropylene, Nanocor Inc. and New Castle
Industries Inc.
36
Kaempfer D, Thomann R, Mülhaupt R, Polymer, 43 (2002) 2909
37
National Institute of Standards and Technology, Gilman JW, Kashiwagi T, Morgan AB, Harris RH Jr, Brassell L, VanLandingham M, Jackson CL, Flammability Of Polymer Clay Nanocomposite
Consortium: Year One Annual Report, http://fire.nist.gov/bfrlpubs/fire00/art026.html (accessed January 2004)
38
Manias E, Touny A, Wu L, Strawhecker K, Lu B, Chung TC, Chemistry Of Materials, 13 (2001) 3516
39
Moussaif N, Groeninckx G, Nanocomposite Workshop, Development of Polymer Nanocomposites by Reactive Extrusion (September 2001) Risley Hall, Derby, UK
40
Karger-Kocsis J, Polypropylene: Structure, Blends And Composites Vol. 3, Chapman and Hall, London, 1995, quoted in ref. 38
41
Karian HG, Handbook Of Polypropylene And Polypropylene Composites, Marcel Dekker, New York, 1999, quoted in ref. 38
42
Gilman JW, Jackson CL, Morgan AB, Harris R, Manias E, Giannelis EP, Wuthenow M, Hilton D, Philips SH, Chemistry Of Materials, 12 (2000) 1866, quoted in ref. 38
43
Theng BKG, Formation And Properties Of Clay-Polymer Complexes, Elsevier, Amsterdam, 1979, quoted in ref. 38
44
Theng BKG, Chemistry Of Clay-Organic Reactions, Wiley, New York, 1974, quoted in ref. 38
45
Kojima Y, Usuki A, Kawasumi M, Okada A, Fukushima Y, Kurauchi TT, Kamigaito O, Journal
Of Materials Research, 8 (1993) 1179, quoted in ref. 38
46
Kojima Y, Usuki A, Kawasumi M, Okada A, Kurauchi TT, Kamigaito O, Journal Of Polymer
Science Part A-Polymer Chemistry, 31 (1993) 983, quoted in ref. 58, 38
47
Jimenez G, Ogata N, Kawai H, Ogihara T, Journal Of Applied Polymer Science, 64 (1997) 2211, quoted in ref. 58
48
Ogata N, Kawakage S, Ogihara T, Journal Of Applied Polymer Science, 66 (1997) 573, quoted in ref. 58
49
Ogata N, Jimenez G, Kawai H, Ogihara T, Journal Of Polymer Science Part B-Polymer
Physics, 35 (1997) 389, quoted in ref. 58
50
Akelah A, Moet A, Journal Of Materials Science, 31 (1996) 3589, quoted in ref. 58
51
Tudor J, Willington L, O’Hare D, Royan B, Chemical Communications, (1996) 2031, quoted in ref. 58
52
Von Werne T, Patten TE, Journal Of the American Chemical Society, 121 (1999) 7409, quoted in ref. 58
53
Bergman JS, Chen H, Giannelis EP, Thomas MG, Coates GW, Chemical Communications, (1999) 2179, quoted in ref. 58
54
Liu LM, Qi ZN, Zhu XG, Journal of Applied Polymer Science, 71 (1999) 1133, quoted in ref. 58
55
Burnside SS, Giannelis EP, Chemistry Of Materials, 7 (1995) 1597, quoted in ref. 58
56
Vaia RA, Jandt KD, Kramer EJ, Giannelis EP, Chemistry Of Materials, 8 (1996) 2628, quoted in ref. 58
57
Kawasumi M, Hasegawa N, Kato M, Usuki A, Okada A, Macromolecules, 30 (1997) 6333
58
Alexandre M, Dubois P, Sun T, Garces JM, Jérôme R, Polymer, 43 (2002) 2123
59
Wolf D, Fuchs A, Wagenknecht U, Kretzschmar B, Jehnichen D, Häussler L, Proceedings Of The Eurofiller 99, 1999, Lyon-Villeurbanne Pg. 6, quoted in ref. 38
60
Kim KN, Kim H, Lee JW, Polymer Engineering And Science, 41 (2001) 1963
61
Reichert P, Nitz H, Klinke S, Brandsch R, Thomann R, Mülhaupt R, Macromolecular Materials
62
Solc J, Nichols K, Galobardes M, Giannelis EP, SPE ANTEC Technical Papers, 43 (1997) 1931, quoted in ref. 60
63
Hasegawa N, Okamoto H, Kato M, Usuki A, Journal Of Applied Polymer Science, 78 (2000) 1918
64
Vaia RA, Giannelis EP, Macromolecules, 30 (1997) 7990, quoted in ref. 58, 38
65
Vaia RA, Giannelis EP, Macromolecules, 30 (1997) 8000, quoted in ref. 38
66
Balazs AC, Singh C, Zhulina E, Lyatskaya Y, Macromolecules, 31 (1998) 8370, quoted in ref. 38
67
Balazs AC, Singh C, Zhulina E, Lyatskaya Y, Accounts Of Chemical Research, 8 (1999) 651, quoted in ref. 58
68
Reichert P, Hoffmann B, Boch T, Thomann R, Mülhaupt R, Friedrich C, Macromolecular Rapid