Part II
Results
Chapter 2
New synthetic approaches to alk-1-enyl
sulfoxides
2.1 Introduction
The literature analysis reported in the previous part demonstrated that alk-1-enyl sulfoxides can be conveniently synthesized starting from sulfur derivatives and reagents bearing an alkenyl functional group.82,175,176,177 This approach simplifies the synthesis of these compounds, affording the products in high yields under practical conditions. Sulfonyl and sulfinyl chlorides are among the most readily accessible sulfur derivatives, and their utilization in the synthesis of unsaturated sulfoxides represents an obvious choice. Sulfinyl chlorides are in the correct oxidation state for the synthesis of sulfoxides, and thus seem reasonable starting reagents; sulfonyl chlorides, on the other hand, require a reduction step to afford sulfoxides. However, the high reactivity and the moisture sensitivity of sulfinyl chlorides limits their utilization in synthesis. Both sulfinyl and sulfonyl chlorides have been employed successfully in the synthesis of unsaturated sulfoxides during the course of this thesis work. The results obtained, as well as differences in the reactivity of the two substrates, are discussed in the present chapter. Sulfonyl chlorides were studied first in consideration of their widespread availability and their low cost, whereas sulfinyl chlorides were employed only later, and were particularly useful since their reactivity explained some mechanistic details which had the subject of the study on the reactivity of sulfonyl
chlorides. The discussion on the reactivity of sulfinyl chlorides will be dealt with in Section 2.4.
Organoaluminum derivatives, although somewhat underemployed in organic chemistry, represent an interesting class of organometallic reagents, and show their full synthetic potential in the transfer of unsaturated chains. Dialkyl alk-1-enyl alanes are easily accessible in nearly quantitative yields from the corresponding alkynes by the well known hydroalumination reaction, which occurs with complete (E) stereoselectivity; with respect to reactivity, the unsaturated moiety at Al often shows a remarkable reactivity and is transferred preferentially over the alkyl groups. Surprisingly, as shown in the literature analysis, use of these derivatives in the synthesis of unsaturated sulfoxides has been neglected, the sole exception being represented by scattered reports;159 this thesis work was thus mainly devoted to the study of the reactivity of alanes in the preparation of unsaturated sulfoxides. The present chapter deals with the use of these compounds as alkenylating agents vs. sulfonyl and sulfinyl chlorides.
2.2 Syntesis of alk-1-enyl sulfoxides using aluminum
sulfinates
2.2.1 General
remarks
It is well known since the 1960s that aluminum arylsulfinates react with trialkyl- and triaryl alanes giving many structurally different aryl alkyl or diaryl sulfoxides in high yields. This reaction, illustrated in Scheme 2.1, has been extensively studied by Reinheckel et al.,178,179,180 and represents an economical and high-yielding approach to sulfoxides. The reaction proceeds through the in
situ formation of the intermediate aluminum aryl sulfinate 15. This compound is
obtained from the corresponding aryl sulfonyl chloride by treatment with equimolar amounts of triethyl aluminum or diethyl aluminum chloride at room temperature, working at high concentration. Addition of a symmetrical alane gives the desired aryl sulfoxides 16.
Ar S Cl O O Ar S OAlEt2 O Ar S R O Et3Al r.t. 5min. AlR3 15 16 + EtCl + R 2Al O AlR2
Scheme 2.1: Reinheckel protocol for the synthesis of sulfoxides using aluminum sulfinates and alanes
The method can be performed in rather short times (30 minutes) at room temperature. This protocol has, however, a major limitation: alkyl sulfonyl chlorides do not react cleanly; it was in fact reported that the alkylaluminum sulfinates obtained are completely unreactive towards the alanes in the second step of the process, and after hydrolysis the alkyl sulfinic acid is obtained. This feature of the reaction limits the scope of this protocol to the preparation of sulfoxides bearing at least one aryl substituent. The importance of alkenyl sulfoxides prompted to test whether this approach could be extended to the use of alkenyl alanes; the possibility of developing a one-pot procedure made this method even more attractive. The main problem involved in the extension to dialkyl alkenyl alanes may be, in comparison to trialkyl or triaryl alanes,178,179,180 any incomplete selectivity in the transfer of the unsaturated residue.
2.2.2 Preliminary
experiments
Preliminary experiments were performed using experimental conditions similar to those employed by Reinheckel,180 following the procedure reported in Scheme 2.2. In particular, the synthesis of the aluminum sulfinate was performed in dichloromethane solution, at high concentration (1-1.5 M). The (E)-di-i-butyl hex-1-enyl aluminum, prepared by hydroalumination of 1-hexyne, was added in stoichiometric ratio to the homogeneous solution of the aluminum sulfinate.
S Cl O O S OAlEt2 O S O Bu-n Et3Al r.t. 5min. (i-Bu)2Al Bu-n + EtCl + (i-Bu) 2Al O Al(Bu-i)2
Scheme 2.2: Synthesis of alkenyl sulfoxides using aluminum sulfinates
The product was isolated in a disappointing yield (26%, Table 2.1 entry 1), i.e. lower than the yields reported by Reinheckel. This is due to the scarce reactivity of the dialkyl alk-1-enyl aluminum, which is considerably lower than that of trialkyl and triaryl alanes. Neither refluxing the reaction nor the addition of 1 extra equivalent of di-i-butyl hex-1-enyl aluminum improved the yield.
The experiment was repeated at higher concentrations (4M in dichloromethane) and using 3,3-dimethyl but-1-enyl aluminum (entry 2). The yield was also rather disappointing, and did not improve even after 10 hours of reflux, or after addition of 0.5 extra equivalents of organometallic reagent.
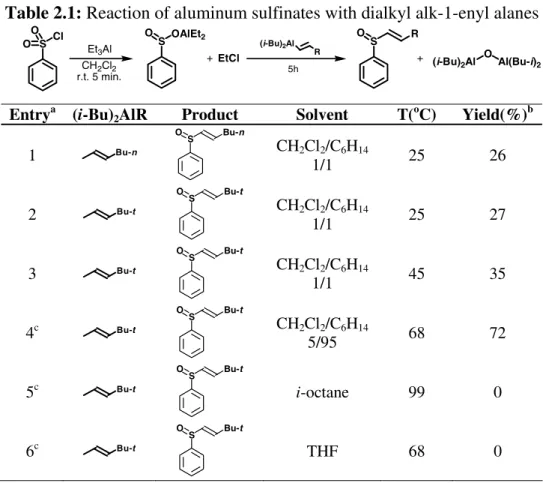
Table 2.1: Reaction of aluminum sulfinates with dialkyl alk-1-enyl alanes
S Cl O O S OAlEt2 O S O R Et3Al CH2Cl2 r.t. 5 min. (i-Bu)2Al R 5h
+ EtCl + (i-Bu)2Al O Al(Bu-i)2
Entrya (i-Bu)
2AlR Product Solvent T(oC) Yield(%)b
1 Bu-n S O Bu-n CH2Cl2/C6H14 1/1 25 26 2 Bu-t S O Bu-t CH2Cl2/C6H14 1/1 25 27 3 Bu-t S O Bu-t CH2Cl2/C6H14 1/1 45 35 4c Bu-t S O Bu-t CH2Cl2/C6H14 5/95 68 72 5c Bu-t S O Bu-t i-octane 99 0 6c Bu-t S O Bu-t THF 68 0
a All the reactions were performed using an (i-Bu)
2AlR/PhSO2Cl=1/1 ratio if not
otherwise stated; b Evaluated on the isolated, chemically pure product and referred to
the sulfonyl chloride; c Reaction performed using an (i-Bu)
2AlR/PhSO2Cl=1.5/1 ratio.
An experiment was carried out by refluxing the reaction mixture immediately after the addition of the alane (entry 3). Under these conditions the product was isolated in considerably higher yield than in the previous experiments. This suggested that an increase of the reaction temperature could enhance the yield. To increase the temperature, a reaction was performed using a solvent mixture enriched in hexane (95%), together with a 1.5/1 alane/aluminum sulfinate ratio; the product was obtained in 72% yield (entry 4). When, however, an even higher temperature (99 oC) was used, performing the reaction in i-octane, no trace of the desired product was detected in the reaction mixture (entry 5). This may be due to the thermal decomposition of the alane.181
In further experiments, use of THF as solvent was studied considering that: 1. Ether-complexed alanes can be prepared more readily than the
corresponding unsolvated analogues; in particular, their synthesis starting from Grignard reagents is very practical;
2. Synthetic interest in the reaction would be greater if ether-complexed alanes could be employed; the choice of solvent, as well as functional group tolerance, would be greater.
The reaction was performed at the boiling point of the solvent, at a temperature similar to that employed in entry 4, in which hexane was employed as solvent. In this case (entry 6) no trace of the desired sulfoxide was obtained, even after prolonged reaction times.
It is tempting to conclude from the above experimental results that the effect of the solvent in this reaction is correlated with its Lewis basicity. The other critical factor is the reaction temperature, i.e. an optimum temperature must be selected so that the alane does not decompose but is activated enough to react with the Al sulfinate. Entries 4 and 6 in Table 2.1, where the reaction temperature is quite similar but yields are remarkably different, clearly show the influence of the Lewis basicity of the solvent. In the light of these results, it is probable that the higher reactivity of trialkyl and triaryl alanes which, according to Reinheckel, smoothly react even at room temperature, is due to their greater Lewis acidity. An interesting feature emerging from this preliminary study is the complete transfer selectivity: the unsaturated sulfoxide was always the sole sulfur-containing product detected in the reaction mixtures; the lower than quantitative yields are probably due to incomplete alane reaction. The unreacted alane and aluminum sulfinate are likely lost in the aqueous work-up, after quenching, thus preventing an accurate mass balance.
2.2.3 Study of the reaction
Once reasonable conditions were found for the reaction under study, attention was directed toward the generalization of the procedure on different substrates. With this aim, the reactivity of different dialkyl alk-1-enyl alanes and sulfonyl chlorides was examined, using the same reaction conditions reported in Table 2.1, entry 4.
The results obtained in this study are summarized in Table 2.2. The reaction proceeds in similar yields and reaction times regardless of the organometallic
reagent, as long as the latter is unsubstituted at the carbon α to aluminum (Table 2.2, entries 1-5). Interestingly, yields obtained employing methanesulfonyl chloride as starting material are quite similar to those observed when aryl sulfonyl chlorides are used (Table 2.2, entry 1 vs. 5).
Table 2.2: Alk-1-enyl sulfoxides using aluminum sulfinates
R' S Cl O O R' S OAlEt2 O R' S O R Et3Al CH2Cl2 r.t. 5 min. (i-Bu)2Al R Hexane, rfx. 5h (1.5 equiv.) +EtCl + (i-Bu) 2Al O Al(Bu-i)2 Entrya (i-Bu)
2AlR R’SO2Cl Product Yield(%)b
1 Hex-n S Cl O O O S Hex-n 75 2 Hex-c S Cl O O OS Hex-c 75 3 Bu-n S Cl O O OS Bu-n 75 4 Bu-t S Cl O O Me S Me O Bu-t 72 5 Bu-n Me S Cl O O Me S O Bu-n 75 6 Et Et S Cl O O OS Et Et 0
a All the rections were performed using an (i-Bu)
2AlR/R’SO2Cl=1.5/1 molar
ratio at the temperature of 68 oC; b Calculated on the isolated, chemically pure
product and referred to the sulfonyl chloride.
These results show that reaction conditions found during this work are efficient for the synthesis of alk-1-enyl sulfoxides starting from alkyl sulfonyl chlorides, in contrast to what reported by Reinheckel. The main limitation of this reaction is the failure to obtain alkynyl sulfoxides starting from dialkyl alk-1-ynyl alanes. In these cases no trace of product could be recovered even after prolonged reaction times.
In conclusion, this reaction represents a major extension over the Reinheckel protocol, allowing the synthesis of aryl as well as alkyl alk-1-enyl sulfoxides. The reaction is easy to perform and leads to satisfactory yields. Two main features make this protocol particularly attractive: first, selectivity in the transfer of the
unsaturated chain was always complete: GLC-MS and NMR analyses did not show any trace of alkyl transfer byproducts in any of the reaction mixtures. The excellent selectivity of the transfer process allows an extremely simple hydrolysis procedure: it is in fact sufficient to adsorb the reaction mixture on silica gel and elute with dichloromethane to obtain the pure product; the second feature consists in the extremely high level of stereoselectivity both in the hydroalumination step and in the subsequent transfer of the unsaturated chain; this allows formation of the stereochemically pure (E)-isomers from each reaction.
2.3 Alk-1-enyl
sulfoxides
starting from sulfonyl
chlorides and pyridine-coordinated alanes
2.3.1 Preliminary attempts of cross-coupling reactions in the
presence of palladium complexes
The literature analysis in the preceding part discussed an interesting synthetic pathway to alk-1-enyl sulfones.77 This approach, shown in Scheme 2.3, is a cross-coupling reaction between alkenyl stannanes and sulfonyl chlorides, catalyzed by palladium complexes. As discussed in the previous section, there are some doubts about the mechanism of this reaction, and the possibility of a radical mechanism cannot be ruled out. Despite these considerations, the method seemed attractive in view of the simplicity of the procedure and the good yields obtained. With a slight modification of this approach, alk-1-enyl sulfones could perhaps be obtained starting from sulfonyl chlorides and dialkyl alk-1-enyl alanes, which in turn are obtained by hydroalumination of alkynes. The advantage of alanes vs. organostannanes is the lower toxicity and simpler synthesis of the former.
R S Cl O O R=Ar, Me n-Bu3Sn R' + Pd(PPh3)4 70 oC, 15min R S O O R' + n-Bu3SnCl 60-90%
Scheme 2.3: Reaction of sulfonyl chlorides with organostannanes in the presence of palladium catalysts
Preliminary attempts were carried out in the presence of PdCl2(PPh3)2 or
Pd(PPh3)4, using CH2Cl2 as solvent. Under these reaction conditions both
triethylaluminum and, after longer reaction times, (E)-di-i-butyl hex-1-enyl aluminum, afforded aluminum sulfinate as the sole product (Scheme 2.4).
Formation of this product was established by isolating the sulfinic acid after acidic aqueous work-up. This reaction is in good agreement with what reported by Reinheckel on the formation of aluminum sulfinates.
S Cl O O S OAlR2 O AlR3 "Pd", r.t., 15 min H2O S H O O CH2Cl2 + EtCl
Scheme 2.4: Attempted cross-coupling between organoalanes and sulfonyl chlorides
These results clearly suggest that the uncatalyzed reaction between trialkyl alanes and sulfonyl chlorides is too fast to allow for Pd catalysis. Under these conditions, the Pd catalyst was not active enough to oxidatively add to the sulfonyl chloride, thereby starting the catalytic cycle; the preferred reaction pathway was the formation of the aluminum sulfinate, even when dialkyl alk-1-enyl alanes were employed, although in this case small amounts (20%) of (E) p-tolyl hex-1-enyl sulfoxide and the corresponding sulfone were detected, in a nearly 1/1 ratio. Other experiments were carried out employing THF as solvent, with the aim of forming an alane-THF complex and thereby reduce the reactivity of the alane. Complexes of alanes have lower Lewis acidity, and are expected to be less reactive in the formation of the aluminum sulfinate.
THF S O O Cl + AlEt3 "Pd" S O S O +Et 2Al O AlEt2 17
Scheme 2.5: Cross-coupling reaction between triethyl aluminum and benzenesulfonyl chloride conducted in THF
The exploration of a variety of experimental conditions did not significantly improve the yield; the major change in the outcome of the reaction was represented by the formation of appreciable amounts (75%) of S-phenyl-benzenethiosulfonate 17, as reported in Scheme 2.5. This is in good agreement with previous reports182 on the formation of S-aryl-arylthiosulfonates by action of LiAlH4 in mild conditions, which is reported to occur via formation of intermedite
aluminum sulfinates.183 On the basis of such results, the effect of pyridine as solvating agent for alanes was examined; this may modulate their reaction course. Depending on the order of addition of the reagents, one could obtain two effects:
1. Complexation of alanes with pyridine could moderate their reactivity. It is well documented in the literature that alane-pyridine complexes show a lower reactivity,184 due to lower Lewis acidity.185,186
2. Sulfonyl chlorides could react with pyridine, and this could result in the activation of such intermediate towards substitution at sulfur. This reaction pathway is precedented in the chemical literature.187
As described in more detail later, both effects can operate in this reaction, depending on the reaction conditions. Activation of sulfonyl chloride with pyridine and subsequent alkylation by uncomplexed organoalanes and Grignard reagents in the synthesis of sulfones will be discussed in Section 3.2.
As far as reaction of complexed alanes is concerned, the preformed pyridine-triethyl aluminum complex, was completely unreactive towards benzenesulfonyl chloride both using THF and CH2Cl2. However, the (E) di-i-butyl hex-1-enyl
aluminum/pyridine complex 18, whose formation was evidenced by NMR analysis (Table 2.6), reacted with tosyl chloride in the presence of a catalytic amount (3 mol %) of Pd(PPh3)4, affording the unsaturated sulfone 19 in 48% yield
(Scheme 2.6). Two remarkable facts were observed during this reaction: the triphenylphosphine present as ligand on the palladium catalyst was completely oxidized to triphenylphosphine oxide, and an appreciable amount of p-tolyl hex-1-enyl sulfoxide 20 was also isolated. The sulfoxide was isolated in nearly equimolar amounts vs. the triphenylphosphine originarily present (Scheme 2.6).
S O O Cl + (i-Bu)2Al Bu-n Pd(PPh3)4 Hexane/THF 1:1 N Me S O O Me Bu-n 48% 3h r.t. + PPh3O Me 9% + 12% S O Bu-n 18 19 20
Scheme 2.6: Reaction of di-i-butyl hex-1-enyl alane-pyridine complex and tosyl chloride in the presence of Pd(PPh3)4
Further experimentation showed that addition of further triphenylphosphine to the reaction mixture led to the immediate formation of more sulfone 19, and concomitant appearance of alkenyl sulfoxide 20 was also observed. Formation of
20 could be ascribed to a direct reaction between sulfonyl chloride, the
the triphenylphosphine. A control experiment was carried out using stoichiometric amounts of triphenylphosphine and no palladium. As shown in Scheme 2.7, many products were isolated, the major ones being (E)-hex-1-enyl p-tolyl sulfone 19, (E)-hex-1-enyl p-tolyl sulfoxide 20 and i-butyl p-tolyl sulfoxide. The formation of the unsaturated sulfone clearly demonstrated that the palladium catalyst acted only as triphenylphosphine source, and that sulfone was produced by non-Pd pathway.
S O O Cl + (i-Bu)2Al Bu-n Hexane/THF r.t., 15min N Me S O O Me Bu-n + PPh3O S Me Bu-n + O PPh3 + S Bu-i Me O 18 19 20
Scheme 2.7: Reaction of tosyl chloride and di-i-butyl hex-1-enyl alane in the presence of stoichiometric amounts of triphenylphosphine
The reaction temperature was then lowered to 0 oC to increase the selectivity of the process; under these conditions the unsaturated sulfoxide 20 was the sole product detected; it was recovered in a 48% yield. This fact suggested that sulfone
20 is not intermediate in the formation of sulfoxide. It reasonable to assume that,
were the reduction of sulfone 19 to sulfoxide 20 to take place as the second step in the reaction, a lower temperature should disfavor or in any case slow the process down, and thus a higher yields of sulfone should be observed. To definitively prove that sulfone was not an intermediate, a reaction was performed adding a known amout (35% mol/mol) of an authentic sample of sulfone 19 to the preformed alkenylalane-pyridine complex 18. After the addition of the other reagents (0.9 equivalents of tosyl chloride and 1.2 equivalents of triphenylphosphine) and of the appropriate internal standard, the reaction progress was monitored by GLC. The sulfone was effectively reduced by triphenyl phosphine under these reaction conditions, but the reduction was quite slow, requiring several hours, in contrast with the few seconds that are sufficient to perform the main reaction; this suggested that formation of sulfoxide proceeded through another pathway. In addition, simply mixing sulfone and triphenylphosphine did not lead to the formation of sulfoxide nor to the oxidation of triphenylphoshpine (68 oC, 24h). From the above data, it was reasonable to assume that sulfone and sulfoxide were formed by two different pathways. Both processes were investigated in detail; the results obtained in the synthesis of
sulfoxide are discussed below, whereas the synthesis of sulfone will be considered in Section 3.3.
2.3.2 Alk-1-enyl sulfoxides from pyridine-complexed alanes and
sulfonyl chlorides in the presence of triphenylphosphine
On the basis of the preliminary experiments described in the previous section, the effect of temperature, reagent ratio and solvent was examined. The results are reported in Table 2.3. A major factor is the nature of the solvent employed (entries 8-10), while stoichiometry and temperature play a marginal, although not negligible, effect (entries 1-7).
Table 2.3: Effect of reaction parameters on the yield
S Cl O O (i-Bu)2Al Bu-n N Me + PPh3 S Bu-n O Me + Ph3PO + (i-Bu)2Al N Cl 0 oC 10min Entry Pyridinea TsCla PPh 3a Solvent Yield(%)b,c 1 1 0.9 0.9 THF/Hexane 1/1 50 2 1 2 2 THF/Hexane 1/1 23 3 1 0.9 0.9 THF/ Hexane 1/1 43d 4 1 1.35 0.9 THF/ Hexane 1/1 61 5 1 0.45 0.45 THF/ Hexane 1/1 42 6 2 0.9 0.9 THF/ Hexane 1/1 59 7 2 1.8 0.9 THF/ Hexane 1/1 64 8 1 0.9 0.9 Hexane 23 9 1 0.9 0.9 THF 83e 10 1 0.9 0.9 CH2Cl2 87
a Molar ratio with respect to the organometallic reagent; b Calculated on the
isolated, chemically pure product, based on the limiting reagent between the alane and the sulfonyl chloride; c Maximum yield was usually achieved
within 10 min from the addition of triphenylphosphine; d Reaction
temperature was -30 oC; e The reaction took 3h to go to completion
From the collected data, we conclude that solvents endowed with Lewis base properties, e.g. THF, reduce the reaction rate but maximize the yield (entries 9-10
vs. 8); it is also evident that CH2Cl2 is the best solvent among the ones tested, and
it was used in all the subsequent reactions. In order to improve the yields further, the reaction was then optimized performing a chemiometric analysis on the system. The chemiometric analysis is increasingly employed in chemistry, because it allows one to achieve a complete or nearly complete description of the system studied by performing only a limited number of experiments.188 Basically,
a chemiometric approach considers the system studied as a multivariate system, in which one or more parameters have to be maximized by varying a number of different independent variables. The interesting feature of this approach is that it allows, even in the case of complex systems, to locate the absolute maximum of the variable examined, and not a local maximum, which is often found employing a univariate method (i.e. varying the reaction parameters one at time). Moreover, a complete description of the system is achieved, which allows one to gain a better control of the reaction.
Table 2.4: Chemiometric analysis of the sulfoxide formation reaction
S Cl O O (i-Bu)2Al Bu-n N Me + PPh3 S Bu-n O Me CH2Cl2 0 oC Entrya (i-Bu) 2AlR TsClb PPh3b Yield(%)c 1 1 1 1.6 62 2 1 1.18 1.52 69 3 1 1.2 1.2 72 4 1 1 1.1 71 5 1 0.8 1.2 83 6 1 0.78 1.52 71 7 1 1.2 1.4 60 8 1 0.8 1.4 93 9 1 1.02 1.4 91.5 10 1 0.98 1.42 88 11 1 1.02 1.42 91
aAll the reactions were performed at 0 oC in CH 2Cl2; b
Molar ratio referred to the alkenyl alane; c Measured
using internal standard (n-nonadecane)
A multivariate fitting analysis afforded the following values for the coefficients of the previous equation:
2 1 2 2 2 1 2 1 9.05 22.84 15.15 5.77 5 . 5 94 . 89 X X X X X X Y = − − − − +
The R-squared value was found to be 0.986, thus indicating an excellent fitting of the experimental values. Graphical representation of the equation obtained is given in Figure 2.1, and clearly shows the dome-like shape of the function: Yield = f(PPh3,TsCl); the axes in the figure represent the values of the parameters
Figure2.1: Contour representation of the function obtained with the chemiometric analysis. PPh3/alane ratio (1.10-1.60) is reported on the X axis;
TsCl/alane ratio (0.83-1.17) is reported on the Y axis; both ratios were normalized in the range (-1;1)
The analysis of the fitting function allows one to identify the experimental conditions for maximum yield. On the basis of the theoretical results a dialkyl alk-1-enyl alane/tosyl chloride/triphenylphosphine = 1/0.92/1.35 ratio would give the best yield, which was estimated to be 91%±4 at the 95% confidence level. A reaction using this optimized ratio afforded the pure product in an excellent 94% yield, in good agreement with predictions.
It was interesting to examine whether, and to what extent, the optimized conditions for the synthesis of (E)-p-tolyl hex-1-enyl alane were general for the synthesis of different substrates. It seemed unlikely that such finely tuned reaction conditions could be optimal for the synthesis of structurally different substrates. Reactions were conducted employing the same optimized reaction conditions found before, and as shown in Table 2.5, yields of all products are similar, provided that the stereoelectronic requirements of the system are not too different from those of the chosen model.
Noteworthy is the result reported in entry 6; triphenylphosphine was completely oxidized, but only a modest yield (35%) of the desired sulfoxide was observed. The low yield observed in this case is presumably due to a reaction between the acidic protons α to the sulfonyl moiety and pyridine present in the reaction mixture. This hypothesis seems to be confirmed by what reported in the literature on reactions between amines and sulfonyl chlorides.189 The relatively unreactive dialkyl alk-1-ynyl alanes are completely unreactive towards sulfonyl chlorides also in this reaction, analogously to what observed in Section 2.2.3.
Table 2.5: Alk-1-enyl sulfoxides starting from pyridine-complexed alanes and
sulfonyl chlorides in the presence of triphenylphosphine R' S Cl O O (i-Bu)2Al R N + 1.35 PPh3 R' S R O CH2Cl2, 0 oC 10min 0.92 + Ph3PO + (i-Bu)2Al N Cl Entrya (i-Bu)
2AlR R’SO2Cl Product Yield(%)b
1 Hex-n S Cl O O Me S Me O Hex-n 94 2 Hex-c S Cl O O Me S Me O Hex-c 92 3 Bu-t S Cl O O Me S Me O Bu-t 89 4 Bu-n S Cl O O S Ph O Bu-n 70 5 Ph S Cl O O Me S Me O Ph 75 6 Bu-n Me S Cl O O Me S O Bu-n 35c
a All reactions were conducted in CH
2Cl2 at 0 oC employing a ratio
(i-Bu)2AlR/R’SO2Cl/PPh3 of 1/0.92/1.35; b Calculated on the isolated,
chemically pure product; c Yield did not increase even after 24 h.
2.3.3 Mechanistic
details
The attention was then directed at the analysis of the mechanistic details of the reaction under study. The very fast kinetics of the reaction made the direct observation of reaction intermediates problematic, but some important observations could be made:
1. The intermediacy of alk-1-enyl sulfones en route to the corresponding sulfoxide can be confidently ruled out. As stated before, a reaction carried out adding a known amount of sulfone to the reaction mixture proved that sulfone is reduced only at a remarkably slower rate. It is clear that phosphine reduces preferentially and almost selectively the sulfonyl
chloride, which in fact disappears from the reaction mixture almost instantaneously. The presence of a Lewis acid is necessary to reduce the sulfone, since in the absence of alanes the sulfone could not be reduced (68
oC, 48 h).
2. The formation of sulfinyl chloride by reduction of sulfonyl chloride with triphenylphosphine and pyridine, represented in Scheme 2.8, can be excluded too. S Cl O O S Cl O S O R PPh3 (i-Bu)2Al R + Ph3PO + (i-Bu)2AlCl + Ph3PO
Scheme 2.8: Possible reaction pathway involving a sulfinyl chloride
As will be more extensively explained in Section 2.4, benzenesulfinyl chloride reacts with both uncomplexed and pyridine-complexed dialkyl alk-1-eyl alanes affording a very complex reaction mixture, as represented in Scheme 2.9. The alkenyl sulfoxide is detectable only in traces. On the other hand, when triphenylphosphine oxide is employed as catalyst, alkenyl sulfoxides are obtained in fairly good yields (35-57%). However, under these conditions pyridine-complexed alk-1-enyl alanes fail to give the sulfoxide, affording instead appreciable amounts of S-S coupling products, mostly 21 and 22.
(i-Bu)2Al R + Ph S Cl O Ph S S O O Ph S S + CH2Cl2 0 oC N Ph Ph 21 22
Scheme 2.9: Reaction between alanes and sulfinyl chlorides
3. As stated above, sulfinyl chlorides afford aryl alk-1-enyl sulfoxides in the presence of catalytic or stoichiometric amounts of triphenylphosphine oxide, and it is thus probable that some common intermediate exists in these two reactions.
The presence of free sulfinyl chloride in the examined reaction is however unlikely, due to the high reactivity of this compound towards pyridine-complexed and unpyridine-complexed alanes; the key intermediate in the synthesis of sulfoxides is likely to be somewhat less reactive that a sulfinyl chloride.
On the basis of the above observation, a resonable mechanism is depicted in Scheme 2.10. In this proposal, after the complexation of unsaturated alane 23 with pyridine, which affords intermediate 24, addition of sulfonyl chloride would lead to complex 25. Addition of triphenylphosphine to this intermediate would lead to the formation of 26 by substitution of pyridine on the sulfur center, and to its isomer 27, in which the phosphorus atom is bound to the oxygen of sulfonyl chloride. Final substitution of triphenylphosphine oxide by the alkenyl chain present on the alane would bring about formation of the sulfoxide.
(i-Bu)2Al R N 24 (i-Bu)2Al R Py RSO2Cl Ar S N O O (i-Bu)2Al R 25 PPh3 Ar S O (i-Bu)2Al 27 O R PPh3 S O Ar R Ph3PO + + Cl Cl (i-Bu)2AlCl 23 Ar S PPh3 O O (i-Bu)2Al R 26 Cl
Scheme 2.10: Proposed reaction pathway for the synthesis of alk-1-enyl sulfoxides starting from pyridine-alane complexes, triphenylphosphine and sulfonyl chlorides
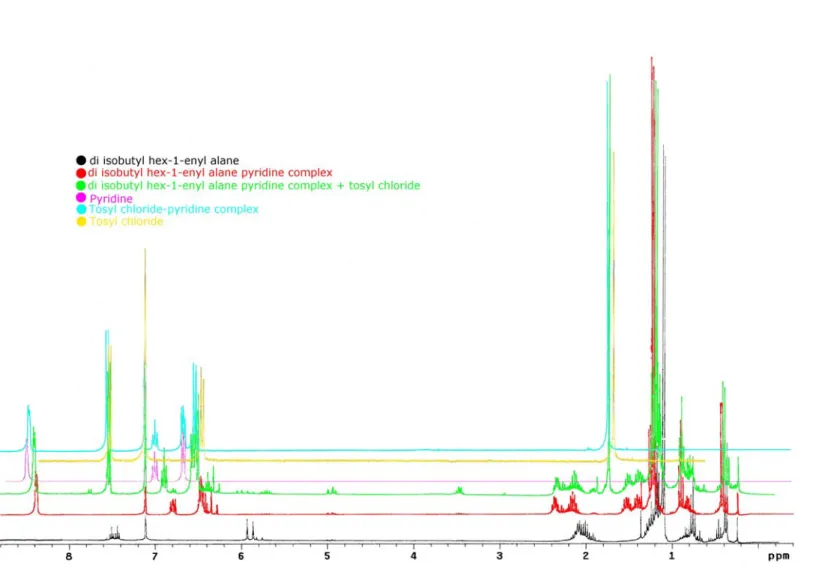
NMR studies were performed to find the intermediates in the reaction and confirm the mechanistic hypothesis advanced. As shown in Figure 2.2, when a stoichiometric amount of pyridine is added to a benzene solution of the di-i-butyl hex-1-enyl alane (Scheme 2.10, compounds 23 and 24), a dramatic change in the chemical shifts of the alane and pyridine protons is observed (Figure 2.2, black and red spectra). This confirms the formation of some kind of alane-pyridine complex. The pyridine bound to the alane can be freed upon addition of a stronger ligand; it was in fact verified that addition of the more basic 4-(N,N-dimethyl)-aminopyridine (DMAP) causes decomplexation of pyridine and formation of a new DMAP-di-i-butyl hex-1-enyl aluminum complex. This complex is unreactive in the above reaction.
Upon addition of tosyl chloride, noticeable changes occur in chemical shifts of tosyl chloride, pyridine and di-i-butyl hex-1-enyl aluminum, suggesting formation of a pyridine-tosyl chloride complex still bound to the alane (Figure 2.2, green spectrum); the presence of a simple pyridine-tosyl chloride complex can be ruled out by the absence of signals characteristics of uncomplexed alane. Moreover, the tosyl chloride-pyridine complex shows remarkably different chemical shifts from those of the species present in the solution (Figure 2.2, green vs. blue spectra). The most plausible structure is depicted in Scheme 2.10, compound 25, which is in good agreement with the spectroscopic evidence. A synoptical view of the 1H chemical shifts for the different species is shown in Table 2.6.
After addition of triphenylphosphine, the reaction proceeds too quickly to be monitored by NMR, and all the structures proposed in Scheme 2.10 should be regarded as speculative. However, intermediate 27, if present, is likely to afford the sulfoxide; it is in fact reasonable to assume that reactivity of compound 27 is similar to that of the intermediate 28 formed when sulfinyl chloride is reacted with triphenylphosphine oxide; as stated before and shown in Scheme 2.11, compound 28 affords sulfoxides in fairly good yields.
S O Cl Ph Ph3PO + Ar S O O (i-Bu)2Al R Cl S O Ar R Ph3PO + +(i-Bu)2AlCl 45-57% 28 PPh3
Scheme 2.11: Reaction between sulfinyl chlorides, uncomplexed alanes and triphenyl phosphine oxide
The proposed mechanism succeeds in the explanation of some experimental features of the reaction. In particular, complete oxidation of triphenylphosphine and disappearance of sulfonyl chloride is always observed even when alkenyl sulfoxide does not form. It is evident from the mechanism depicted in Scheme 2.10 that intermediate 27 affords, after hydrolysis, sulfinic acid and triphenylphosphine oxide; these are in fact the sole products recovered in the case in which the alane is not able to transfer the unsaturated chain to the sulfur center. The proposed mechanism requires decomplexation of the alane from the nitrogen ligand in the very first step of the reaction.
This is in good agreement with the observation that using DMAP as ligand shuts down the reaction. It was verified by NMR that DMAP is able to quantitatively substitute pyridine in the complex with the alane; it is likely that
this complex is too stable to react with sulfonyl chloride, and this inhibits the first step of the reaction (formation of compound 25, Scheme 2.10). On the other hand, heteroaromatic derivatives with basicity similar to that of pyridine (e.g. isoquinoline, 4-ethyl pyridine) afford the sulfoxides in identical yields to those observed when pyridine is employed. When triethylamine is used as ligand, the reaction affords a nearly equimolar amount of sulfone and sulfoxide, in low overall yield (25%). It should be noted that this result is similar to that obtained when unsolvated alanes were used; triethylamine is known as a weak ligand for alanes, due to the steric hindrance of the ethyl chains. Likely, the formed complex is too labile to effectively moderate the reactivity of the alane, which reacts as its uncomplexed form.
Table 2.6: Chemical shifts (expressed in Hertz) of the species evidenced in
Scheme 2.10 Al a a a a b b c c d e f g h i N S Cl O O l l m m n o o p p q
Proton compoundsFree a i-Bu2AlR + Pya i-Bu2AlR + Py + TsCla TsCl + Pya
a 330.3 364 353 - b 601 646 636 - c 111.5 126 117 - d 1770 1895 1884 - e 2242 1935 1929 - f 629 708 699 - g 345-390 405-480 390-465 - h 345-390 405-480 390-465 - i 227 269 265 - l 2543 2513 2514 2538 m 2002 1942 1950 2000 n 2101 2038 2061 2099 o 2258 - 2260 2263 p 1936 - 1960 1959 q 503 - 522 521.5
a All spectra were recorded on a Varian Infinity spectrometer, at the frequency of 300
MHz. The signals were referred to the residual signal of C6H6, set exactly at 2134.36 Hz.
Hz were used instead of the more common ppm to better evidence even small changes in chemical shifts.
In conclusion, this new synthetic approach to aryl alk-1-enyl sulfoxides starting from sulfonyl chlorides, pyridine-alane complexes and triphenylphosphine affords
the products in excellent yields, often greater than 90%. This reaction can be performed one-pot, under very mild conditions using inexpensive reagents; the reagents are in simple stoichiometric ratios, and this provides an efficient utilization of the organometallic reagent; this should be compared with the synthesis of alk-1-enyl sulfoxides with aluminum sulfinates, described in Section 2.2, which required an alane/sulfinyl chloride molar ratio of 1.5/1, and thus yields based on the organometallic reagents were about 50%. As for the previous reaction, the process is completely stereoselective, affording pure (E)-isomers.
2.4 Alk-1-enyl sulfoxides starting from sulfinyl
chlorides
2.4.1 General
remarks
As already discussed, sulfinyl chlorides can be considered as the most direct precursors to alk-1-enyl sulfoxides, because the sulfur atom is already in the appropriate oxidation state. This feature allows the synthesis of unsaturated sulfoxides without the need for a reducing agent. Sulfinyl chlorides are unfortunately very reactive, and due to their sensitivity toward water it is necessary to store and handle them under inert atmosphere.
Ph S O Bu-n CH2Cl2, r.t. Ph S Cl O 15% Ph S S O O S S + (i-Bu)2Al Bu-n + 30min Ph Ph + 21 22 + (i-Bu)2AlCl Ph
Scheme 2.12: Reaction between benzenesulfinyl chloride and di-i-butyl hex-1-enyl aluminum
The use of such compounds was taken into consideration despite the limitations involved with their use; it was in fact of interest to try to gain access to alk-1-enyl sulfoxides by a pathway different from the reductive ones examined above. A study of the reactivity of sulfinyl chlorides vs. alanes may be useful in explaining some mechanistic details of the previous reaction. As discussed in Section 2.3.3, one may want to consider the possibility that sulfinyl chlorides were formed during the course of the reaction.
CH2Cl2, r.t., 30min Ph S Cl O Ph S O O Cl 28 PPh3 Ph S O Bu-n (i-Bu)2Al Bu-n 52% 29 PPh3O
(1 equiv.) +(i-Bu)2AlCl+ Ph3PO
Scheme 2.13: Reaction between benzenesulfinyl chloride and di-i-butyl hex-1-enyl alane in the presence of Ph3PO
A reaction was first performed by reacting benzenesulfinyl chloride with unsolvated (E)-di-i-butyl hex-1-enyl aluminum. The corresponding sulfoxide was recovered in a very modest yield (15%) and S-phenylbenzenethiosulfonate 21 and of diphenyl disulfide 22 were detected (Scheme 2.12).
It is clear from this preliminary experiment that sulfinyl chlorides are too reactive to afford the sulfoxide in good yields. It is in fact possible that sulfinyl chloride reacted in the presence of Lewis acids by a radical pathway to give the homocoupled product; this would be not unprecedented in the chemistry of these sulfur derivatives. Pyridine-coordinated alanes also gave disappointing results, leading to a mixture of S-S coupling products and affording the sulfoxides only in traces. This could be explained by postulating a ligand exchange in solution, resulting in the activation of the sulfinyl chloride towards the undesired reaction. Taking into account the mechanistic proposal discussed in Section 2.3.3, it is likely that the sulfinyl chloride may react with triphenylphosphine oxide, affording intermediate 28 (Scheme 2.13). This reaction would support the presence of intermediate 27 en route to sulfoxides.
To verify this assumption, the benzenesulfinyl chloride-triphenylphosphine oxide complex was formed, and its formation was confirmed by 31P NMR spectroscopy through the observation of a 3.6 ppm upfield shift of the P signal with respect to triphenylphosphine oxide; when di-i-butyl hex-1-enyl aluminum was added, sulfoxide 29 was obtained in 52% yield (Scheme 2.13) as the only identifiable product. The unreacted sulfinyl chloride is likely lost during the workup as sulfinic acid, and therefore an accurate mass balance was not carried out.
In addition to supporting the mechanistic hypothesis advanced in Section 2.3.3, this reaction represented an interesting approach to the synthesis of aryl alk-1-enyl sulfoxides, and it was therefore useful to study the effect of the amount of triphenylphosphine oxide on the reaction yield. The results showed that the use of 0.5-1 equivalent of triphenylphosphine oxide gave uniform yields, i.e. the reaction is, to some extent, catalytic (the oxide is not consumed stoichiometrically) but a
further reduction to 0.25 equivalents causes the yield to drop (32%). Finally, different substrates were tested, to study the scope of this reaction; the results are collected in Table 2.7.
Table 2.7: Sulfoxides starting from benzenesulfinyl chloride and alanes in the
presence of triphenylphosphine oxide R Alk2Al R' S R O S Cl O CH2Cl2 Ph3PO S OPPh3 O CH2Cl2 + Alk2AlCl + Ph3PO Cl Entrya (i-Bu)
2AlR Product Yield(%)b
1 Bu-n S O Bu-n 52 2 Bu-t S O Bu-n 57 3 Bu-t S O Bu-t 43 4 S O 48
a All the reactions were performed at room temperature using
an Alk2AlR/PhSOCl/PPh3O=1/1/0.5 ratio; b Calculated on the
isolated, chemically pure product
As shown in Table 2.7, yields are usually around 50%. However, sulfinyl chlorides react with moisture, and this feature makes it rather difficult to quantify the conversion, which presumably is not quantitative. This hypothesis is supported by the fact that no byproduct was found in any of the reaction mixtures. This approach is the only method among those reported in this study which can be successfully employed to synthesize aryl alk-1-ynyl sulfoxides in acceptable yield. As already stated, dialkyl alk-1-ynyl alanes were in fact completely unreactive in all the other approaches examined. Despite the low conversions, the above reaction is particularly interesting due to the lack of byproducts, which simplifies isolation and purification of products. Moreover, the possibility to obtain acetylenic sulfoxides gives an easy access to (Z)-alkenyl sulfoxides, which
can be obtained by one of the reduction methods reported in the literature145 and previously discussed (Section 1.3.1).