3. Results
3.1 In vitro analyses of ACES (Animal Cap Embryonic Stem) cells expressing
different doses of noggin mRNA
3.1.1 High doses of noggin can effectively induce the expression of retinal cell type specific markers in ACES cells
Different doses of noggin mRNA (from 2.5pg to 20pg of mRNA) were microinjected into both blastomeres of Xenopus laevis embryos at two-cell stage, GFP mRNA (400pg) was coinjected as tracer or injected alone as control. At blastula stages (stage 9), animal caps from injected embryos were explanted and in vitro cultured until stage 39 (a stage in which embryonic retinogenesis is almost complete, by reference to sibling wild type embryos). To analyze cell-fate commitment in
noggin-expressing ACES cells, RT-PCR, in situ hybridization and immunohistochemistry were
performed.
RT-PCR revealed that all noggin doses tested (2.5pg to 20pg) were able to activate in ACES cells the expression of general neuronal markers such as Sox2 (Mizuseki et al., 1998) (Fig.3.1.1 A) and of genes expressed in proliferating, undifferentiated retinal precursors such as CycD1, Xotch,
Xash1, NeuroD (Casarosa et al., 2003) (Fig.3.1.1 B). However, only dosages of 15pg or more of noggin mRNA were able to elicit the expression of terminal differentiation markers of specific
retinal cell types, such as Xbh1 and Brn3d (ganglion cells, Poggi et al., 2004), Prox1 (horizontal cells, Onorati et al., 2007), Opsin and NRL (photoreceptors, Whitaker et al., 2004) (Fig.3.1.1 A).
Immunohistochemistry and in situ hybridization experiments confirmed the results of RT-PCR. As shown in Figure 3.1.2, both in 2.5pg- and 20pg-noggin injected ACES cells, the expression of acetylated-tubulin (Ace-tubulin) was activated, demonstrating that noggin-expressing ACES cells were properly neuralized (Black and Keyser, 1987). However, only in high noggin doses-expressing ACES cells, the expression of terminally differentiated retinal cell markers were activated, such as Opsin protein (marker of photoreceptors) in Figure 3.1.2 C, C’, hermes mRNA (marker of ganglions, Gerber et al., 1999) in Figure 3.1.2 D, D’ and vsx1 mRNA (marker of bipolar cells, D’Autilia et al., 2006) in Figure 3.1.2 E, E’.
3.1.2 ACES cells expressing high doses noggin mRNA effectively differentiate into retinal lineages
To determine whether ACES cells at stage 39 have differentiated into cell types other than retinal neurons, we initially analyzed the expression of neuronal forebrain markers in 20pg
noggin-expressing ACES cells, such as Nkx2.4 (a ventral telencephalic marker, Lupo et al., 2002)
and Emx1 (a dorsal telencephalic marker, Pannese et al., 1998) by in situ hybridization, and the expression of neural crest marker, Snail (Linker et al., 2000) by RT-PCR, to check whether noggin can induce neural tissue such as forebrain and neural crests (Kencht and Harland, 1997). In no cases (0/30) the expression of Nkx2.4 and Emx1 was found in the explants (Fig.3.1.3 A, B), and no Snail
transcripts were found in total RNA extracts (data not shown). Moreover, we carried out EdU incorporation and Xotch in situ hybridization, a progenitor marker (Dorsky et al., 1995), to detect proliferating cells, a certain percentage of 20pg noggin-expressing ACES cells was found to be proliferating (Fig.3.1.3 C).
In order to determine the identity of high doses noggin-expressing ACES cells at early developmental stages, the 20pg noggin-expressing ACES cells were cultured and harvested at stage 15, total RNA was extracted and followed by RT-PCR molecular marker analysis. As shown in Figure 3.1.4, 20pg of noggin induce the expression of forebrain markers such as Otx2, BF1 and
Eomes (Lupo et al., 2002), the expression of presumptive neural crest marker Snail (Linker et al.,
2000), and the expression of eye field specific transcription factors such as Rx1 and Pax6, but not the expression of hindbrain marker Krox-20 (Bradley et al., 1993). For negative control, the expression of retinal differentiation markers such as Opsin and Brn3d was not found. These observations suggest that high doses of noggin trigger anterior neural induction, and high dose
noggin-expressing ACES cells at neurula stage can be considered as a tissue reminiscent of anterior
neural tissue in the embryo, which is permissive to develop into forebrain neurons, neural crests and retinal neurons.
To validate the effectiveness of 20pg of noggin in driving ACES cells to differentiate into retinal cell fates, we counted the positive cell numbers of different marker staining and estimated the total cell numbers using Hoechst nuclear staining in the injected explants. Table 1 shows frequencies of marker staining and sample sizes at stage 39. Based on the observation that total percentage of retinal cell types together with proliferating progenitors is almost 100% (that is, 48.75% of N-tubulin-labeled differentiated neurons and 18.17% of Opsin-labeled photoreceptors plus 28.97% of EdU labeled proliferating cells), we can conclude that high doses of noggin can effectively drive ACES cells to commit into retinal lineages at different levels of differentiation. We propose that EdU-labeled or Xotch-positive cells in 20pg-noggin expressing ACES cells can be considered (to some extent) as Müller glia progenitors, since this cell type at stage 39 is not yet fully differentiated (Wong and Rapaport, 2009).
A
Figure 3.1.1 In vitro analysis of noggin injected ACES cells by RT-PCR. A: Multiplex RT-PCR of terminaldifferentiation retinal markers. Only noggin doses higher than 15pg elicit expression of markers of specific retinal cell types in ACES cells. The general neuronal marker
Sox2 expression is activated by all doses of noggin tested, showing that noggin correctly
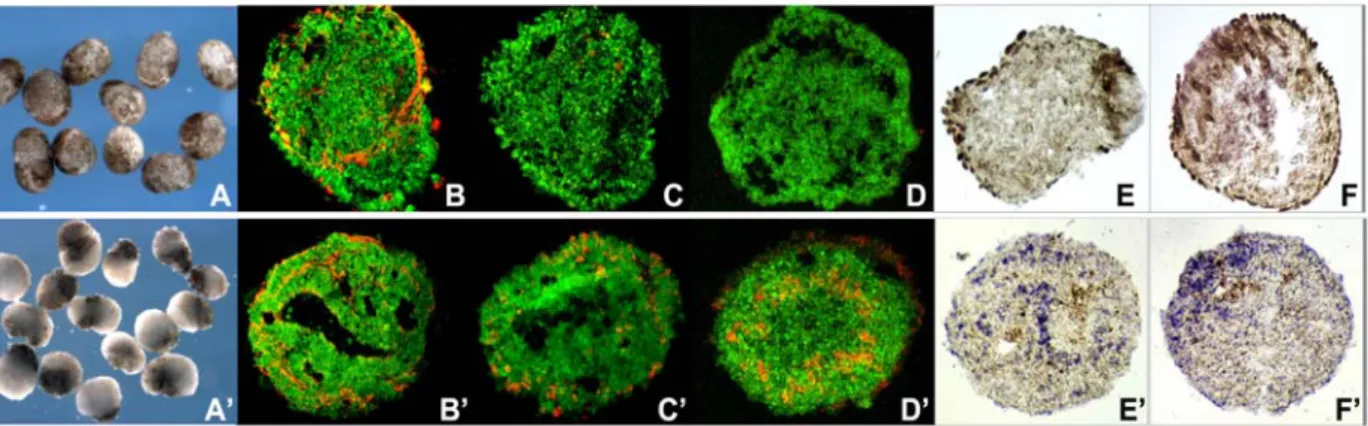
Figure 3.1.2 In vitro analyses of noggin-expressing ACES cells by immunohistochemistry and in situ hybridization. A-F, 2.5pg noggin-expressing ACES cells; A’-F’, 20pg noggin-expressing ACES
cells. A, A’: noggin-treated ACES cells in vitro cultured in 1xMBS medium, they show different aspects. ACES cells expressing low doses of noggin have brown-colored epithelium, but ACES cells expressing high doses of noggin show regionalized pigmentation since they are polarized by
noggin (Kencht and Harland, 1997); B, B’: Ace-tubulin immunostaing (in red); C, C’: Opsin
immunostaing (in red); D, D’: hermes in situ hybridization (in red); E, E’: Vsx1 in situ hybridization (in blue), and F, F’: Xotch in situ hybridization (in blue); GFP expression in green.
Figure 3.1.3 In 20pg noggin-expressing ACES cells, no expression of forebrain markers (A, B)
was found by in situ hybridization and a certain percentage of proliferating cells was detected by EdU incoporation (C, EdU in red, GFP in green).
B
B: Multiplex RT-PCR of retinal progenitorsmarkers. All doses of noggin tested induce the expression of retinal progenitor markers.
Pax2, Vax2 and Zic2 were tested as
molecules implicated in retinal dorso-ventral patterning (Lupo et al., 2006). we: whole embryos; uninj.: non-injected embryos; -RT: negative control, reverse transcriptase was omitted.
Table 1
N-tubulin Differentiated neurons 9/9 5707 2782 48.75 hermes Ganglion cells 12/12 2625 512 19.50
Opsin Photoreceptors 18/18 5211 947 18.17
Vsx1 Progenitors and bipolar cells 9/9 4775 2034 42.60 Xotch Proliferating progenitors 18/18 5008 1622 32.39
EdU Proliferating cells 7/7 4464 1293 28.97 Percentages of 20pg noggin-injected ACES cells expressing the different markers. The total number of cells in each explant was estimated by Hoechst nuclear staining.
3.2 In vivo analyses of ACES cells expressing different doses of noggin mRNA
3.2.1 ACES cells transplantation assay and description of the resulting phenotypes
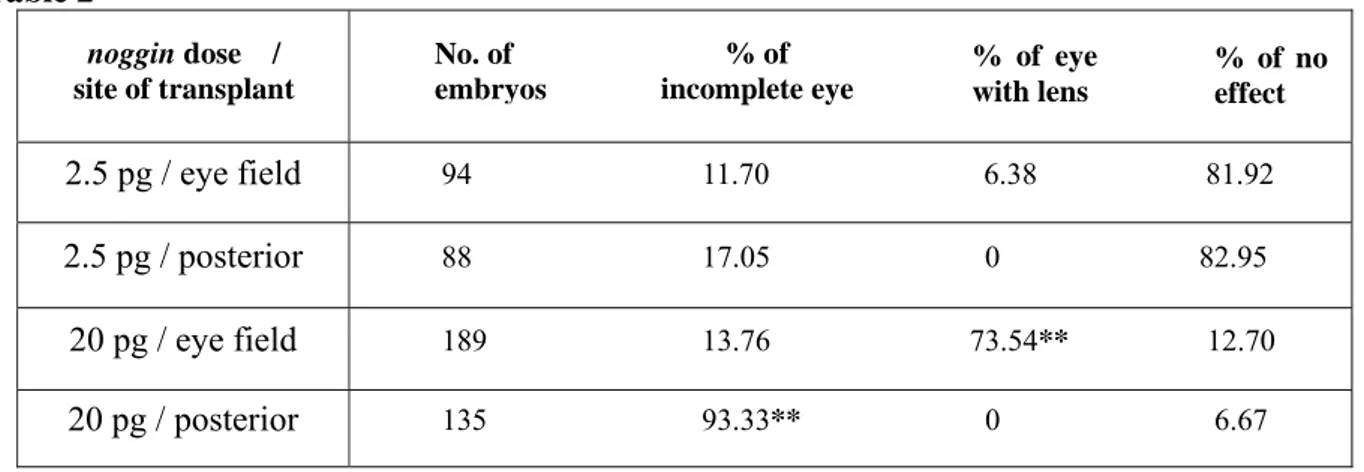
To test the eye-forming potential of noggin-expressing ACES cells in vivo, transplantation assay was performed. We analyzed the effects of 2.5pg or 20pg of noggin-injected ACES cells transplanted in the eye field region and in the posterior neural tube, respectively. The transplantation was performed when the injected ACES cells and sibling embryos reached stage 15. Table 2 and Figure 3.2.1 summarize and compare the resulting phenotypes and their percentages.
No. of positive explant Total No. of cells No. of positive cells % of positive cells Cells labeled Markers
Figure 3.1.4 RT-PCR molecular marker
analysis on 20pg noggin-injected ACES cells harvested at stage 15. GFP st15: GFP-expressing ACES cells used as control; 20 nog st.15: 20pg noggin-expressing ACES cells collected at stage 37; Head st.37: heads from stage 37 WT embryos used as control; -C: cDNA templates were omitted; -RT: reverse transcriptase was omitted in HisH4 reaction.
In all experiments, transplantation of ACES cells in the eye field region was anticipated by removal of eye field on one side of the embryo, leading to the ablation of the eye on the surgical side. In GFP control transplants, GFP-expressing ACES cells can integrate into host embryos. In particular, eye field transplants integrated into the head epidermis (Fig.3.2.1 A), whereas GFP-expressing ACES cells posteriorly transplanted migrated extensively along the tail tissues (Fig.3.2.1 A’). In no cases GFP-expressing ACES cells were detected in the neural tissue (insets in Fig.3.2.1 A,A’), and they never expressed neural markers, such as N-tubulin.
By contrast, both 2.5pg and 20pg noggin-expressing ACES cells were neuralized, no matter when they were cultured in vitro or were grafted into embryos, as they expressed Ace-tubulin or
N-tubulin in the explants (Fig.3.2.2 B,D), respectively. In the transplants of 2.5pg noggin-expressing ACES cells, only a small percentage of embryos exhibited a non-well-developed
eye (6.38% of eye with lens) or pigment condensation (11.70% of incomplete eye, Fig.3.2.2 E) in the anterior eye field location, whilst such eye-like structures were never found in the posterior transplants (Table 2). By contrast, in the transplants of 20pg noggin-expressing ACES, 73.54% of the cases showed the formation of morphologically recognizable eyes with lens, as revealed by expression of β-crystalline, a lens specific marker (Fig.3.2.1 C,D). Strikingly, the formation of an almost complete eye-like structure was frequent also in the posterior transplants (93.33%), even if in this ectopic location a lens was never formed (no expression of β-crystalline, Fig.3.2.1 C’,D’).
Since the specification of eye field occurs as early as gastrulation (around stage 12.5 in the
Xenopus embryo), the induced eye formed in the eye field location could indicate that
noggin-injected ACES cells were driven to form eyes under intrinsic and extrinsic influences from anterior neural tube and underlying mesoderm. More interestingly, eyes formed also in the posterior neural tube, a location far away from the extrinsic signaling driving normal eye formation, strongly suggesting that high doses of noggin itself are able to activate a developmental program that leads exclusively to retinal differentiation, resulting in eye formation in vivo, also in ectopic locations. Moreover, the expression of GFP demonstrates that the induced eyes derive from the
noggin-injected ACES cells. Table 2 2.5 pg / eye field 94 11.70 6.38 81.92 2.5 pg / posterior 88 17.05 0 82.95 20 pg / eye field 189 13.76 73.54** 12.70 20 pg / posterior 135 93.33** 0 6.67 % of incomplete eye % of eye with lens % of no effect No. of embryos noggin dose / site of transplant
Percentages of incomplete and complete eyes (% eye with lens) and no effect obtained following the transplant of noggin-expressing ACES cells. The dose of noggin used to inject ACES cells and the site of transplant is indicated. **, p < 0.001, z-test (vs. no effect).
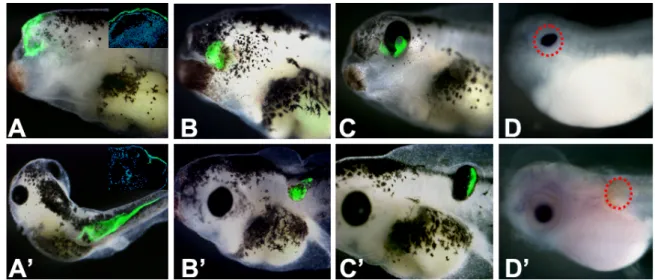
Figure 3.2.1 In vivo analysis of noggin-expressing ACES cells by transplantation assay, a
representative phenotype for each genotype is presented. A-D: eye field transplants; A’-D’: posterior transplants. A, A’: GFP ACES cells. Insets are sections at the level of the transplant site, GFP in green and nuclei in blue, showing incorporation of GFP-expressing ACES cells only in the epidermis; B, B’: 2,5 pg noggin ACES cells, showing no effect of transplants in the host embyos; C,
C’: 20 pg noggin ACES cells, showing the morphologically recognizable eyes formed by ACES
cells; D, D’: in situ hybridization of β-crystalline, a lens specific marker, showing that the induced eyes formed by 20 pg noggin ACES cells in the eye field transplant, but not in the posterior transplant, is an eye complete with lens (the region of induced eye was circled).
3.2.2 Analyses of the noggin-expressing ACES cells in the transplants 3.2.2.1 Histological analysis
Since the incomplete eyes formed by 2.5pg noggin-expressing ACES cells have a poor morphology (as shown in Fig.3.2.3 F,H), we only performed histological analysis on the eyes generated by the transplants of 20pg noggin-expressing ACES cells.
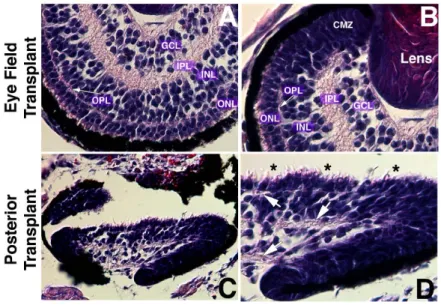
Figure 3.2.2 shows Hematoxylin and Eosin (H&E) staining on paraffin sections of the eyes generated after the transplantation. The induced eye showed a considerable level of organization and structure, not only in the eye field transplants (Fig.3.2.2 A,B) but also in the posterior transplants (Fig.3.2.2 C,D). In the eye field transplants, the eyes exhibited the same histology as normal eyes, as they have morphologically differentiated cell types, a laminated retinal sheet, and fiber layers between the nuclear layers (OPL and IPL in Fig.3.2.2 A,B). In the posterior transplants, even if the overall organization is not perfect, it is clear that cells are organized in nuclear layers divided by fiber layers (arrows in Fig.3.2.2D), and morphological differentiation of photoreceptors
is also evident, even if the outer segment is not well developed, as indicated by asterisks in Figure 3.2.2D.
Figure 3.2.2 Histological analysis (H&E staining) of the 20pg noggin-expressing transplants. A, B: eye field transplants. The resulting eyes show morphologically differentiated cell types, a
laminated retinal sheet, and fiber layers between the nuclear layers. Fiber layer in pink and nuclear layer in blue. C, D: posterior transplants. Even if overall organization is not complete, cells are organized in nuclear layers divided by fiber layers (arrows in [D]) and morphological differentiation of photoreceptors is evident (asterisks in [D]). Abbreviations: CMZ, ciliary marginal zone; GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer.
3.2.2.1 Molecular analysis
To fully characterize the ACES cells transplants, in situ hybridization and immunohistochemistry were both performed to determine cell identities found in transplants. Specific markers for retinal lineages include: Opsin for photoreceptors, Xotx2 for bipolar cells, Glutamine Synthetase for Müller glia, Islet1 for ganglion and amacrine cells, and hermes anti-sense probe for ganglion cells.
GFP-expressing ACES cells only give rise to epidermis both anteriorly and posteriorly (insets in Fig.3..2.1A,A’). However, noggin-expressing ACES cells behaved differently, as they did not show extensive migration as GFP-expressing ACES cells did (especially obvious in the GFP posterior transplants, Fig.3.2.1A’). Conversely, noggin-expressing cells stayed grouped even if they did not form an eye (Fig.3..2.1B,B’). In a small percentage of 2.5pg noggin transplants, which correspond to the transplants showing the formation of incomplete eyes, Opsin expression can be detected in ACES cells both in the eye field transplants (4/5) (Fig.3.2.3E,F) and in the posterior transplants (2/4) (Fig.3.2.3G,H).
consistent with morphological observations. The eyes formed by 20pg noggin-expressing ACES cells always expressed retinal cell type specific markers both in the eye field and in the posterior transplants (Fig.3.2.4). The expression of these markers allowed to highlight the layered structure (except for Glutamine Synthetase, labeling Müller glia that span the whole retina), reminiscent of a WT laminated retina, and the lens was always present in the eye field transplants (Fig.3.2.4A-E), but not in the posterior transplants (Fig.3.2.4A’-E’).
Figure 3.2.3 In vivo analysis of low dose noggin-expressing ACES cells following transplantation
assay. A-D: low dose noggin-expressing ACES cells showing no eye formation in the host embryos are neuralized as revealed by in situ hybridization of N-tubulin (B, D, in blue). E-F: a small percentage of low dose noggin-expressing ACES cells form imcomplete eyes, Opsin expression (in red) was detected in ACES cells both in the eye field transplant (F) and in the posterior transplant (H).
Figure 3.2.4 In vivo analyses of 20pg noggin-expressing ACES cells by immunohistochemistry and in situ hybridization. A-E: analysis of the induced eyes in the eye field transplants; A’-E’: analysis
of the induced eyes in the posterior transplants. A, A’: Opsin immunostaining labeling photoreceptors (in red); B, B’: Xotx2 immunostaing labeling bipolar cells (in red); C, C’: Glutamine Synthetase immunostaing labeling Müller glia (in red); D, D’: Islet1 immunostaining labeling ganglion and amacrine cells (in red); E, E’: hermes in situ hybridisation labeling ganglion cells (in blue); GFP in green. The cellular layers and lens in the eye field transplants are indicated.
3.3 Functional analyses of 20pg noggin-expressing ACES cells transplanted in the
eye field region
Since eyes originating from transplants with ACES cells expressing high noggin doses have a morphology and histology indistinguishable from WT eyes, we investigated whether they were also functional. We performed two different types of analyses.
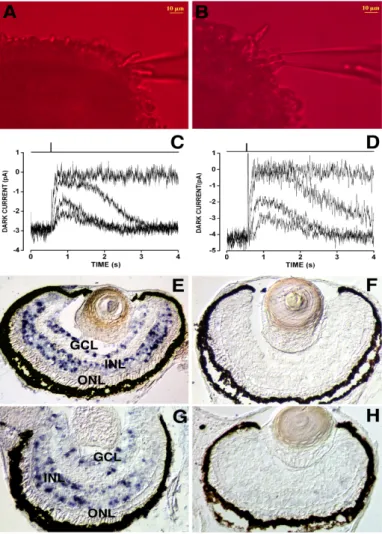
One is electrophysiological recordings of single rod photoreceptors using the suction pipette technique (Baylor et al., 1979), this aims to detect whether the photoreceptors respond to the light. Suction pipette recordings of photoresponse currents on photoreceptors isolated from WT (Fig.3.3A) and 20pg noggin-expressing eye field transplants (Fig.3.3B) were plotted, respectively. We found that they responded identically after a light stimulus (Fig.3.3C,D). Therefore, the photoreceptors in the induced eyes are functional.
We then performed detection of c-fos expression in the retina, this aims to investigate the anatomical and functional integrity of the different layers within the retina. Both WT embryos and 20pg noggin-expressing eye field transplanted embryos were cultivated in 12 hours light/ 12 hours dark (LD12:12) cycle till stage 43, and they were fixed during light or dark, respectively. Then c-fos
in situ hybridization was carried out on retinae of WT (Fig.3.3E,F) and 20pg noggin-expressing eye
field transplants (Fig.3.3G,H) fixed during the light (Fig.3.3E,G) or the dark period (Fig.3.3F,H). It is known that, in the retina of animals grown on a LD12:12 cycle the immediate early gene c-fos is transiently expressed in the inner cell layer (INL) and in the ganglion cell layer (GCL) starting from 30 minutes after the onset of light, while it is completely absent in these layers during the dark period (Richter et al., 1988). This activation can be seen as an indication of the transduction of the light stimulus from the photoreceptors to the second order neurons in the retina. These data show that the retinae originating from the transplants behave identically to WT retinae, that is, activate
c-fos mRNA expression after the onset of light in the INL and GCL, when grown on a LD12:12
cycle.
Taken together, our data show that the eyes originating from the 20pg noggin-expressing eye field transplants are able to respond to light, and are thus functionally indistinguishable from normal eyes.
Figure 3.3 Functional properties of 20pg noggin-expressing eye field transplants. A,B: a rod
photoreceptor outer segment projecting from a retinal fragment is shown inside the recording pipette for either a stage 46 wild type (WT) Xenopus (A) or a time-matched 20pg noggin-expressing eye field transplant (B). Images were acquired under infrared (k>850 nm) light using a cooled charge-coupled device camera (Leica DFC-350FX; Heerbrugg, Switzerland) mounted on an inverted microscope (Leica DMI 4000B). C,D: sweeps plot the time course of the inward dark current suppression by light flashes of increasing intensities for either a stage 46 wt Xenopus (C) or for a time-matched 20pg noggin-expressing eye field transplant (D). Dark current amplitudes (i.e., number of cGMP-gated channels open in the dark) and light sensitivities were similar for wt and transplanted rods. Qualitatively similar results were obtained in additional seven WT and eight transplanted rods. E-H: c-fos in situ hybridization on WT (E,F) and 20pg noggin-expressing eye field transplants (G,H) collected in light (E,G) or dark (F,H). Both WT and transplanted retinae show c-fos expression in the inner nuclear layer and ganglion cell layer only during the light period. Abbreviations: GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer.
3.4 Combination, in ACES cells, of high doses of noggin with transcription factors
that induce specific retinal cell fates
The degeneration of neurons and photoreceptors in the retina that occur in a number of disorders are common causes of blindness for which there are currently few therapies. The repair of the retina with replacement cells derived from human embryonic stem cells could thus provide the
basis for new approaches to treat retinal degenerations (Lamba et al., 2008). As described above, we have successfully induced retinal neurons in vitro by overexpression of high doses of noggin in ACES cells, we then intended to direct these ACES cells to adopt a specific retinal cell fate, especially photoreceptors, for the purpose of cell replacement.
It has been reported that Xotx5b promotes generation of photoreceptors studied by in vivo lipofection (Viczian et al., 2003), and Xotx5b and XNrl both regulate the expression of rhodopsin and reprogram retinal precursors to rod fate in Xenopus (Whitaker et al., 2004; McIlvain and Knox, 2007). In this study, we intended to test the potentiality of these retinal cell fate specific transcription factors together with noggin to drive photoreceptor fate in the ACES cells.
3.4.1 In vitro and in vivo analyses of ACES cells co-injected with high dose noggin and Xotx5b To this end, 20pg of noggin mRNA and 50pg of Xotx5b mRNA were microinjected into both blastomeres of embryos at two-cell stage, injection of 20pg-noggin served as control, GFP mRNA was coinjected as tracer. The injected animal caps were explanted at mid-blastula stage (stage 9). To assess the effects of the combination of noggin and this transcription factor, the resulting ACES cells were in vitro analyzed by immunohistochemistry and in situ hybridization to detect the expression of markers of specific retinal cell fates, and were in vivo analyzed by transplantation assay.
When noggin+Xotx5b-expressing ACES cells were grafted in host embryos, they formed eyes at high efficiency both in the eye field and in the posterior transplants, like noggin-expressing ACES cells did. Figure 3.4.1 shows the expression of retinal cell type specific markers in injected ACES cells and in transplants. All the markers tested are normally expressed both in vivo and in
vitro, as compared with their expression in the 20pg noggin-expressing ACES cells. However,
detailed investigation of Opsin expression by real-time PCR showed obvious increased level of
Opsin mRNA in ACES cells coinjected with noggin and Xotx5b. Counting the Opsin-positive cells
in the noggin+Xotx5b-expressing ACES cells showed the increased number of positive cells when compared with those in 20pg noggin-expressing ACES cells (data not shown). These data suggest combination of high dose noggin and Xotx5b effectively promote photoreceptor fate in ACES cells. To assess whether photoreceptor cells are differentiated precociously by the effect of Xotx5b in
vitro, Opsin immunostaining was detected in the noggin+Xotx5b-expressing ACES cells and in the
WT retina of sibling embryos at serial stages during retinogenesis (from stage 24, proliferating phase, to stage 39, approaching the end of retinogenesis). The Opsin expression was detectable from stage 35 onward in the WT retina (Fig.3.4.2A-C), and this is also the case in the
noggin-expressing and noggin+Xotx5b-expressing ACES cells. Neither noggin (Fig.3.4.2A’-C’) nor noggin plus Xotx5b (Fig.3.4.2A’’-C’’) can precociously activate Opsin expression in vitro. Since
most of rod photoreceptors are post-mitotic at stage 35 in Xenopus retina (Chang and Harris, 1998), the result confirms our expectation that the commercially available Opsin antibody used in our study labels differentiated rod photoreceptors in the Xenopus retina (monoclonal Anti-Opsin, Cat.
No. O4886, Sigma).
Figure 3.4.1 In vivo and in vitro analyses of noggin+Xotx5b-expressing ACES cells by
immunohistochemistry and in situ hybridization. A-H: in vivo analysis of the induced eyes generated from noggin+Xotx5b-expressing ACES cells in the eye field transplants (A-D) and in the posterior transplants (E-H). A and E: Opsin immunostaing labeling photoreceptors (in red); B and F:
Vsx1 in situ hybridization (in blue) and Xotx2 immunostaing (in red) labeling bipolar cells,
respectively; C and G: hermes in situ hybridization labeling ganglion cells (in blue); D and H:
Xotch1 in situ hybridization labeling proliferating cells in the CMZ (in blue). I-L: in vitro analysis
of the noggin+Xotx5b-expressing ACES cells. I: Opsin immunostaining labeling photoreceptors (in red); J: Vsx1 in situ hybridization labeling bipolar cells (in red); K: hermes in situ hybridization labeling ganglion cells (in blue); L: Xotch1 in situ hybridization labeling proliferating cells (in blue); GFP in green.
Figure 3.4.2 Analysis of Opsin expression in the wild-type retina (A-C), 20pg noggin-expressing
ACES cells (A’-C’), and in the noggin+Xotx5b-expressing ACES cells (A’’-C’’) at serial stages. A, A’, A’’: stage 34; B, B’, B’’: stage 35; C, C’, C’’: stage 39.
3.5 Comparison of activities of Noggin and tBR in ACES cells
tBR is a dominant-negative type I BMP receptor (truncated BMP Receptor lacking the cytoplasmic domain, Graff et al., 1994) and is used as a factor to completely block the BMP signaling pathway in the early Xenopus embryos and animal cap explants (Suzuki et al., 1994; Sasai et al., 1995; Suzuki et al., 1997; Massé et al., 2004). Like Noggin, tBR overexpression in ectodermal explants can induce neural fate (Sasai et al., 1995)
To investigate whether Noggin induces retinal cell fates by antagonizing BMP signaling, we employed tBR to inhibit BMP signaling and then compared its effect to that of noggin.
3.5.1 Activity of tBR in Xenopus embryos
In order to have similar levels of activity of the in vitro transcribed tBR mRNA with noggin mRNA, tBR coding region was PCR amplified from the previous construct of p64T-tBR (Suzuki et al., 1994) and subcloned into pCS2 vector, which is the same vector harboring noggin cDNA. tBR mRNA at different doses (from 25pg to 400pg) was microinjected in the ventral marginal zone of one blastomere of 4-cell stage embryos to check its in vivo activity.
It has been shown previously that embryos ventrally overexpressing tBR developed secondary axis by inhibiting the normal BMP signaling that exists in the ventral marginal mesoderm (Suzuki et al., 1994) . At a dose of 100pg, tBR effectively induced formation of the secondary body axis (69%, n = 78, arrows in Fig.3.5.1). A small percentage of embryos showing no effect might be due to a mistaken dorsal injection (Suzuki et al., 1994). It should be noted that, differently from noggin overexpression, none of tBR-overexpressed embryos (n = 308) developed head structures, such as cement gland or eyes, in the anterior part of the secondary axis.
3.5.2 Cell identities in the tBR-expressing ACES cells
In order to test whether tBR plays a dose-dependent activity in ACES cells like noggin does, different doses of tBR mRNA (from 100pg to 1600pg) were microinjected into both blastomeres of two-cell stage embryos. Injected animal caps expressing different doses of tBR mRNA were
Figure 3.5.1 tBR overexpression in Xenopus embryos. Xenopus embryos
microinjected with 100pg of tBR mRNA in the ventral marginal region developed secondary axis (arrows) without any head structures.
isolated and harvested at stage 37, then followed by Opsin immunohistochemistry to determine the commitment of photoreceptors in ACES cells. None of these doses can induce the Opsin expression, but all the tested doses can properly neuralize ACES cells as revealed by Ace-tubulin immunostaining (Fig. 3.5.2 D). Therefore, a dose-dependent activity of tBR can be excluded.
To compare the activities of tBR and noggin in ACES cells, 800pg of tBR mRNA were microinjected into the animal pole of both blastomeres of two-cells stage embryos, and the injected animal caps were explanted at mid-blastula stage. The expression of retinal differentiation markers in the injected explants and the performance of implanted ACES cells in the host embryos were compared between tBR-expressing and noggin-expressing ACES cells.
Interestingly, we found that ACES cells expressing tBR mRNA behave similar to ACES cells expressing low doses of noggin in some aspects. Particularly, both 800pg of tBR and 2.5pg of
noggin can properly neutralize the ACES cells in vitro as shown by Ace-tubulin immunostaining
(Fig. 3.5.2 D and Fig. 3.1.2 B), and can induce the cement gland-like structures in the ACES cells (shown by arrows in Fig. 3.5.2 A,B, and C: in situ hybridization staining of XCG1, a specific marker of cement gland). Finally, in no cases eyes were formed from tBR-expressing ACES cells after transplantation into the host embryos, but these tBR-expressing cells stay grouped on the transplants (Fig. 3.5.2 E,F). Such behavior is similar to 2.5pg of noggin-expressing cells exhibited when they did not form any eye-like structures on the transplants (Fig.3..2.1B, B’).
Moreover, RT-PCR molecular marker analysis revealed that, in ACES cells in vitro cultured till stage 37, both 2.5pg of noggin and 800pg of tBR activate the expression of the general neural marker Sox2 and of the retinal progenitor marker Rx1, but not retina terminal differentiation markers such as Opsin and Brn3d (Fig. 3.5.3). The expression of Otx2 was also found both in 2.5pg
noggin-expressing and 800pg tBR-expressing ACES cells. This observation is in accordance with a
model in which BMP signaling and Otx2 work together to promote cement gland formation (Gammill and Sive, 2000). In addition, 2.5pg of noggin and 800pg of tBR trigger the expression of forebrain makers BF1 and eomes, and the expression of the neural crest marker Snail, but not the expression of the posterior neural marker Krox-20 (Fig. 3.5.3), indicating these ACES cells have been specified, besides to cement gland, to anterior neural tissues at late embryonic stage.
The cement gland is a mucus-secreting organ in the most anterior portion of Xenopus embryos, and it is considered that a low level of inhibition of BMP signaling results in the induction of cement gland (Knecht and Harland, 1997). We used 2.5pg of noggin as a low dose, so that the induction of cement gland tissue is expected and it is an indication of antagonism of the BMP signaling at a low level. Unexpectedly, the induction of cement gland by 800pg (even higher as 1600pg) of tBR mRNA might suggest that tBR can not block the BMP signaling completely. We thus think that our attempt to completely block BMP signaling by tBR ectopic overexpression did not completely succeed. We think it is not so surprising since there is a possibility that unknown BMP type I receptors exist in Xenopus but have not been cloned yet, and another possibility is that induction of retinal cell fates by high doses noggin might employ other types of BMP receptors.
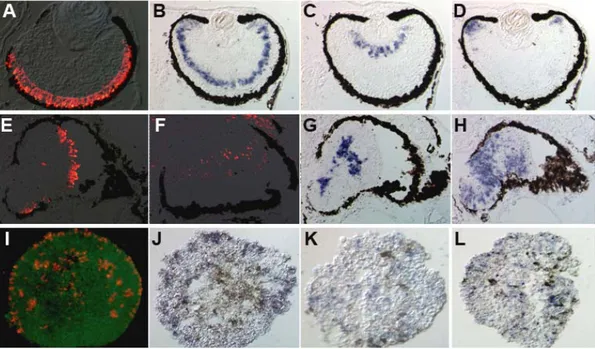
Figure 3.5.2 The phenotypes produced by injection of 800pg tBR are similar to those obtained with
2.5pg noggin in ACES cells. A-D: represent sections of injected ACES cells; A: 2.5pg
noggin-expressing ACES cells; B: XCG1 in situ staining (in blue) on ACES cells expressing 2.5pg
of noggin to reveal the cement gland-like structure; C: ACES cells expressing 800pg of tBR, arrows in A and C point out the cement gland; D: Ace-tubulin immunostaining (in red, GFP in green) on ACES cells expressing 800pg of tBR; E and F: in vivo analysis of tBR-expressing ACES cells by transplantation assay showing that no eye is formed in the host embryos, E: eye-field transplant, F: posterior transplant, GFP in green.
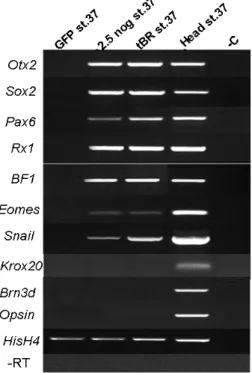
Figure 3.5.3 RT-PCR molecular marker
analysis on ACES cells injected with
noggin or tBR mRNA. GFP st37:
GFP-expressing ACES cells used as control; 2,5 nog st.37: 2.5pg
noggin-expressing ACES cells collected at
stage 37; tBR st.37: 800pg tBR-expressing ACES cells collected at stage 37; Head st.37: heads from stage 37 WT embryos used as control; -C: cDNA templates were omitted; -RT: reverse transcriptase was omitted in HisH4 reaction.
3.6 Synergistic effects of noggin and Shh signaling on the retinal cell fate induction
Microarray anaysis comparing GFP-, 2.5pg noggin- and 20pg noggin-expressing ACES cells (that will be described elsewhere) show involvement of Shh, Wnt and BMP signaling pathways in Noggin-mediated retinal induction.
It has been known that hedgehog (Hh) family of molecules affects multiple aspects of retinogenesis (see Sakagami et al., 2009 for review). In early neurogenesis, Shh derived from first-born RGCs promotes the progression of ganglion cell differentiation wave, but suppresses RGC genesis as these neurons accumulate (Zhang and Yang, 2001). Shh signals also appear to influence the growth and trajectory of RGC axons (Sanchez-Camacho and Bovolenta, 2008). Furthermore, laminar organization of the retina is disrupted in Shh mutants (Wang et al., 2002; Shkumatava et al., 2004). At much earlier stage, however, the role of Shh in eye field specification has not been reported yet except that Shh is involved in the separation of the single eye field into two bilateral fields (Li et al. 1997). Our microarray data show that components of Shh pathway, such as Smoothened, Gli2 and Zic2, are upregulated in ACES cells expressing 20pg of noggin. It is possible that Shh signaling is activated as a downstream event of Noggin action in the ACES cells and plays a synergistic role with noggin in induction of retinal cell fates in ACES cells. In this study, we have tested this hypothesis by the strategies of transplantation assay combined with the treatments of antagonist of Shh signaling.
In an attempt to test whether the activation of Shh signals is necessary to induce the retinal cell fates in ACES cells, 20pg noggin-expressing ACES cells were isolated from injected embryos at blastula stage and cultured in medium containing 50μM of Cyclopamine (an inhibitor of Shh signaling, Decembrini et al., 2009). At stage 15, eye field transplants were made by these treated ACES cells (ACES cells were washed twice with clean 1xMBS solution before transplantation).
We found that Shh signaling is necessary to promote the retinal cell fates in ACES cells. First, by transplantation assay, we compared the eye-forming potential of 20pg noggin-expressing ACES cell treated with or without Cyclopamine. The efficiency of eye formation by 20pg
noggin-expressing ACES cells treated with Cyclopamine is decreased when compared with
untreated noggin-expressing ACES cells (Fig.3.5.4). Secondly, by real time PCR, we validated the effectiveness of Cyclopamine treatment. The expression of Zic2, a Shh downstream target, was significantly decreased in explants treated with Cyclopamine (date not shown). In addition, we analyzed the expression of the EFTFs in the treated or untreated 20pg noggin-expressing ACES cells at stage 15. We found that most of the EFTFs, such as Rx1, Pax6 and Lhx2, are down-regulated in the presence of Cyclopamine (data not shown). Finally, by RT-PCR molecular marker analysis, we compared the pattern of gene expression in the 20pg noggin-expressing ACES cells treated with or without Cyclopamine at stage 37. We found that in the presence of Cyclopamine, the expression of EFTFs such as Pax6 and Rx1 are repressed, and the expression of retina terminal markers such as Brn3d and Opsin are down-regulated, but the expression of Snail is
activated (Fig.3.5.5). The observations on gene expression are consistent with the results of the transplantation assay, that is, repression of EFTFs and retinal terminal differentiation genes results in the decrease of eye-forming potential. Moreover, activation of Snail expression strongly suggests that blocking Shh signaling counteracts the inhibition of BMP signaling by high doses noggin in the ACES cells. Therefore, the evidence provided here leads us to propose that Shh signaling pathway plays a synergistic role with high doses of noggin in driving retinal cell fates.
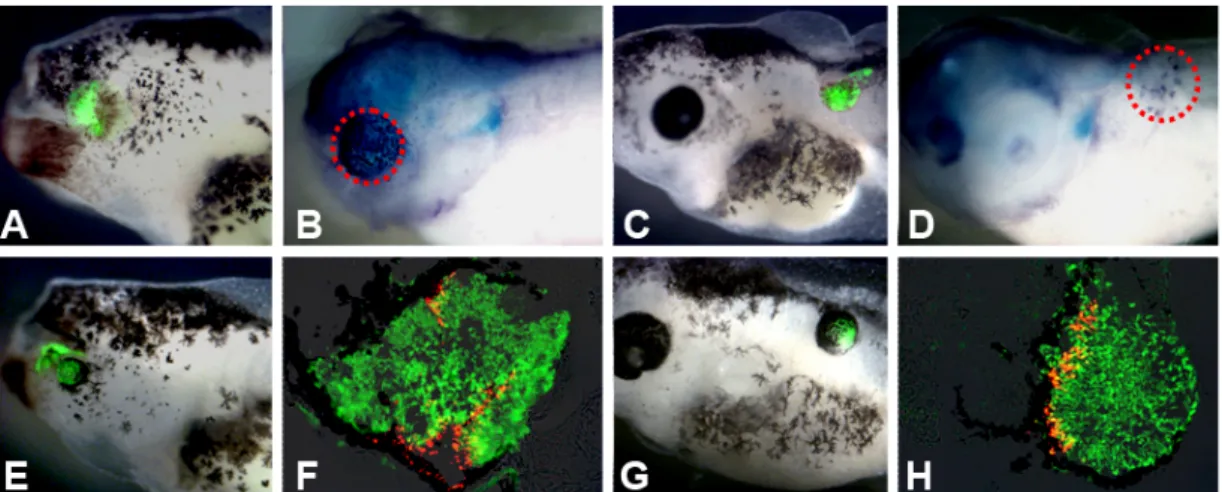
Figure 3.5.4 In vivo analysis of 20pg noggin-expressing ACES cells treated with Cyclopamine
(100μM) till stage 15 by transplantation assay. A: eye field transplant of 20pg noggin-expressing ACES cells showing rescued eye (14/16); B: eye field transplant of 20pg noggin-expressing ACES cells treated with Cyclopamine showing pigment condensation (4/20); C: eye field transplant of 20pg noggin-expressing ACES cells treated with Cyclopamine showing no eye formation (16/20).
Figure 3.5.5 RT-PCR molecular marker
analysis on 20pg noggin-expressing ACES cells treated with or without Cyclopamine (50μM). GFP st37: GFP-expressing ACES cells used as control; 20 nog + Cyclopamine: 20pg
noggin-expressing ACES cells treated
with Cyclopamine and collected at stage 37; 20 nog st.37: 20pg noggin-expressing ACES cells collected at stage 37; Head st.37: heads from stage 37 WT embryos used as control; -RT: reverse transcriptase was omitted in HisH4 reaction.