Chapter 3
New synthetic approaches to unsaturated
sulfones
3.1 Introduction
During the preliminary studies on the synthesis of alk-1-enyl sulfoxides with pyridine-coordinated alanes and triphenylphosphine (Section 2.3.1), appreciable amounts of sulfones were obtained as byproducts. Other experiments proved that the sulfone was not produced by the palladium-catalyzed cross-coupling reaction, but it was formed by a different mechanism (Scheme 2.7). Direct reaction between sulfonyl chloride and dialkyl alk-1-enyl alanes could also be excluded because, as already described in Section 2.2, this reaction leads mainly to the formation of aluminum sulfinates.
It was reasonable to hypothesize the presence of two possible pathways:
1. Formation of a sulfonyl chloride-pyridine adduct (compound 30, Scheme 3.1) and subsequent substitution by the organo alane;
2. Formation of a sulfonyl chloride-triphenylphosphine oxide adduct (compound 31, Scheme 3.1) and substitution by pyridine-coordinated alane;
S O PPh3 Cl 31 Me S N Cl 30 Me O O O O
Scheme 3.1: Plausible intermediates in the formation of sulfones starting from sulfonyl chlorides
Both these intermediates were thoroughly investigated, and this chapter deals with the results obtained. The first part will be dedicated to the discussion of reactivity of intermediate 30, and the use of triphenylphosphine oxide as catalyst (through intermediate 31) will be discussed later.
3.2 Alkenyl sulfones starting from sulfonyl
chloride-pyridine complexes
3.2.1 Use of dialkyl alk-1-enyl alanes as alkenylating agents
The first few experiments were performed with the simplest system among those considered in the previous chapter, the sulfonyl chloride-pyridine adduct. It is well known that reaction with heteroaromatic nitrogen ligands, such as pyridine, activate sulfonyl chlorides to nucleophilic substitution at sulfur. During the NMR studies on the reactivity of pyridine-coordinated alanes with sulfonyl clorides (Section 2.3.3), it was demonstrated that a sulfonyl chloride-pyridine- alane complex 25 is formed under mild conditions; unfortunately, this compound turned out to be unreactive, and did not evolve to sulfone even after prolonged reaction times.
A reaction was performed by slowly adding di-i-butyl-hex-1-enyl aluminum 23 to a stirred CH2Cl2 solution of sulfonyl chloride-pyridine adduct 30. Sulfone 19
was isolated in 47% yield; a 50% conversion, based on the recovered sulfonyl chloride, was observed (Scheme 3.2).
S O O N Me Bu-n (i-Bu)2Al + S O Me Bu-n O CH2Cl2, r.t. 47% Cl 23 30 19 + Cl (i-Bu)2Al N
Scheme 3.2: Reaction of di-i-butyl hex-1-enyl aluminum with tosyl chloride-pyridine adduct
An analogous experiment, performed by adding pyridine – alane complexes to tosyl chloride-pyridine adducts, demonstrated the complete inertness of complexed alanes towards sulfonyl chloride-pyridine adducts. Attention was then turned to the more reactive uncomplexed alanes. When the above reaction was carried out adding the alane over a 15 min, increases in yield (68%) and conversion (80%) were observed.
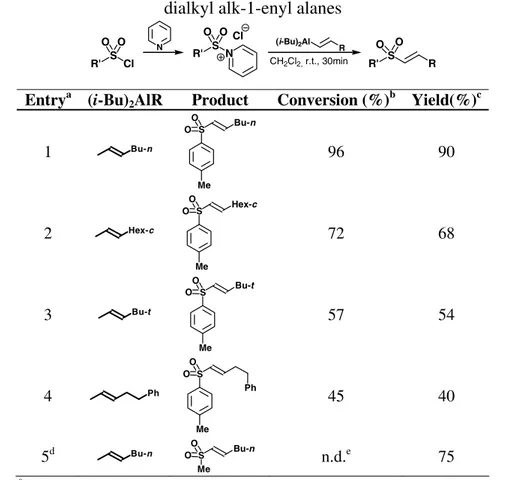
The effect of the addition rate of the organometallic reagent on the yield was studied in several experiments, and it was noted that conversion and yield increased with the addition rate. When fast additions were performed (less than 30 seconds) the product was isolated with a yield of 90% and a conversion of 96%. Similar results were obtained when this procedure was adopted for other alanes with slightly different substituents (Table 3.1).
The conversion seems to depend on the steric bulk of the moiety that is being transferred. Assuming that the less sterically hindered the alkenyl moiety, the more reactive the organometallic compound, one could speculate that alanes are deactivated or consumed by some slow process. Remarkably, the sulfone is always the only isolated product obtained under these conditions. Even in the case of methanesulfonyl chloride, where a discrepancy between conversion and yield is evident, this is presumably due to the work-up, since no product other than the sulfone was observed. Finally, this procedure allows for the preparation of alkyl alk-1-enyl sulfones in synthetically useful yields (Table 3.1, entry 5); these derivatives are not obtainable with the other methods discussed later in this chapter.
Table 3.1: Alk-1-enyl sulfones from sulfonyl chloride-pyridine adducts and
dialkyl alk-1-enyl alanes
R' S Cl O O (i-Bu)2Al R R' S R O O N R' S N O O Cl CH2Cl2, r.t., 30min Entrya (i-Bu)
2AlR Product Conversion (%)b Yield(%)c
1 Bu-n S Me Bu-n O O 96 90 2 Hex-c S Me Hex-c O O 72 68 3 Bu-t S Me Bu-t O O 57 54 4 Ph S Me Ph O O 45 40 5d Bu-n Me S Bu-n O O n.d.e 75
a All reactions were performed by fast addition of the alane to a CH 2Cl2
solution of sulfonyl chloride-pyridine adduct at r.t.; b Based on recovered
sulfonyl chloride; c Calculated on the isolated, chemically pure product; d
Methanesulfonyl chloride was used; e The work-up did not allow the recovery
of the reagent.
3.2.2 The "copper effect"
In our preliminary experiments, it was observed that the Lewis acidity of the alane was an important variable. This was particularly evident from the fact that the use of pyridine completely inhibited the reaction. On this basis, the incomplete conversion of tosyl chloride, observed when slow addition or when sterically hindered alanes were used, could be understood.
+ (i-Bu)2Al Bu-n Me S O O Bu-n Me S N O O + Cl (i-Bu)2Al N Slow + N Me S O O Bu-n (i-Bu)2Al Cl Cl
Scheme 3.3: Ligand equilibrium hypothesized to explain partial conversion in the synthesis of sulfones
Given that when fast additions are performed good conversions are achieved, it is likely that a slow ligand exchange takes place, thus diminishing the concentration of active alane. The postulated equilibrium is represented in Scheme 3.3. It is evident that the concentration of pyridine increases with conversion; when the addition is slow or the reaction of the alane is not fast enough, there is a sufficiently high concentration of pyridine arising from the ligand exchange step to efficiently bind the alane; the complexed organometallic compound is unreactive toward the sulfonyl chloride-pyridine complex, thus hindering conversion.
To overcome this problem, which limited the synthetic utility of the reaction, it was necessary to free the alane from the pyridine originating from the ligand displacement; the simple solution was to find a co-reagent which strongly complexed pyridine, without interfering in other ways with the reaction. Cuprous chloride (CuCl) was chosen for the following reasons:
1. The electronegativity of copper and aluminum is such that transmetallation processes should not be involved.
2. Copper, both in oxidation state I and II, is known to form very stable complexes with pyridine; formation of such compounds would free the alane, thus allowing the reaction to proceed.
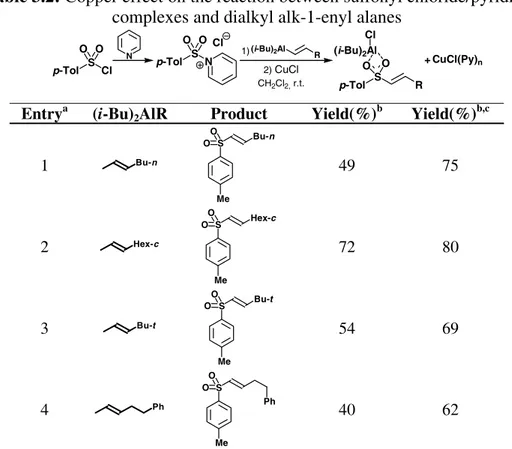
The reactions taken in consideration were the lowest yielding using the general procedure. Any “copper effect” should more evident in these situations, and increase the synthetic utility of the process.
Table 3.2: Copper effect on the reaction between sulfonyl chloride/pyridine
complexes and dialkyl alk-1-enyl alanes
p-Tol S Cl O O (i-Bu)2Al R p-Tol S R O O N p-Tol S N O O Cl CH2Cl2, r.t. 1) 2) CuCl + CuCl(Py)n (i-Bu)2Al Cl Entrya (i-Bu)
2AlR Product Yield(%)b Yield(%)b,c
1 Bu-n S Me Bu-n O O 49 75 2 Hex-c S Me Hex-c O O 72 80 3 Bu-t S Me Bu-t O O 54 69 4 Ph S Me Ph O O 40 62
a All the reactions were performed by adding the organometallic reagent to the
CH2Cl2 solution of the sulfonyl chloride/pyridine complex, allowing the reaction
to reach maximum conversion (2 hours); b By gas chromatography (internal
standard n-nonadecane); c After adding 0.5 molar equivalents of CuCl.
In all cases, the organometallic reagent was added to the stirred solutions of sulfonyl chloride-pyridine adduct, and reactions were stirred until no further conversion was achieved, as monitored by GLC analysis. When this happened, variable amounts (between 0.25 and 0.75 equivalents) of CuCl were then added to the reaction mixture. The appearance of an intense light-blue color was immediately observed in all cases, and after a few minutes (usually 15 minutes) GLC analysis evidenced a remarkable increase in the conversion (Table 3.2). The effect of copper chloride on the reaction could be rationalized with the following facts:
1. A catalytic role of copper can be ruled out. In fact, addition of copper chloride to the reaction mixture immediately prior or after that of the alane led only to the formation of degradation products, mostly arising from the reductive disproportionation of sulfonyl chloride. It is possible that copper chloride may react with sulfonyl chloride, leading to radical species which can be responsible for the observed degradation products. The ability of copper chloride to react with sulfonyl chlorides giving radical intermediates is well documented.90,92
2. Evidence for the complexation of pyridine with copper was obtained from NMR studies of the reaction mixtures. When uncomplexed alanes were mixed with sulfonyl chloride-pyridine complexes, spectra recorded in benzene-d6 solutions showed signals characteristic of the following species: a) sulfonyl chloride-pyridine complex; b) alkenyl sulfone; c) pyridine-alane complex; d) two different di-i-butyl aluminum chloride species, presumably bound to pyridine or to the sulfone. These signals are in excellent agreement with the hypothesis reported in Scheme 3.3. As increasing amounts of copper chloride were added, an increase in conversion was evident; moreover, some broad peaks characteristics of copper-pyridine complexes appeared in the NMR spectra.
From the results presented above, it is tempting to conclude that copper salts interact with pyridine in the reaction mixture. The alane is thus freed to react with the sulfonyl chloride-pyridine complex. In conclusion, the method described herein represents a significant route to alk-1-enyl sulfones. The reaction of uncomplexed dialkyl alkenyl alanes with sulfonyl chloride-pyridine adducts affords the alkenyl sulfones in good yields and transfer selectivity; the conversion can be increased by addition of copper chloride as scavenger for pyridine; as in the case of sulfoxides, the stereoselectivity in the hydroalumination step and in the transfer of the alkenyl chain allows for the preparation of the stereochemically pure (E)-isomer.
3.2.3 Reaction of Grignard reagents with sulfonyl
chloride-pyridine adducts
Considering the good results obtained with alanes in the reaction with sulfonyl chloride-pyridine adducts, it was of interest to examine the use of Grignard reagents in the analogous reaction. It was known from the literature that alkynyl
presence of thionyl chloride,190 whereas alkyl Grignard reagents afford the corresponding symmetrical sulfoxides and sulfides. Surprisingly, little is reported on the reaction of Grignard reagents with sulfonyl chlorides. It is reported that this reaction lacks selectivity, affording mixtures of sulfones, sulfoxides and sulfides, due to the ability of Grignard reagents to reduce the S=O bond. In this brief investigation, attention was directed towards the use of alkynyl and aryl Grignard reagents, in view of their easy preparation and of the synthetic interest of diaryl and aryl alkynyl sulfones. A preliminary reaction was performed adding p-methoxyphenyl magnesium bromide to a THF solution of tosyl chloride-pyridine adduct at room temperature; a satisfactory yield of sulfone (75%) was obtained (Scheme 3.4). S Me Cl O O Py (1 equiv.) THF, r.t. S Me N O O Cl MgBr MeO S Me O O OMe 75% r.t., 30min
Scheme 3.4: Reaction of tosyl chloride-pyridine complex with p-methoxyphenyl magnesium bromide
Differently from what reported with alkyl Grignard reagents, the reaction was quite chemoselective, and no trace of the corresponding sulfide or sulfoxide was detected in the reaction mixture.
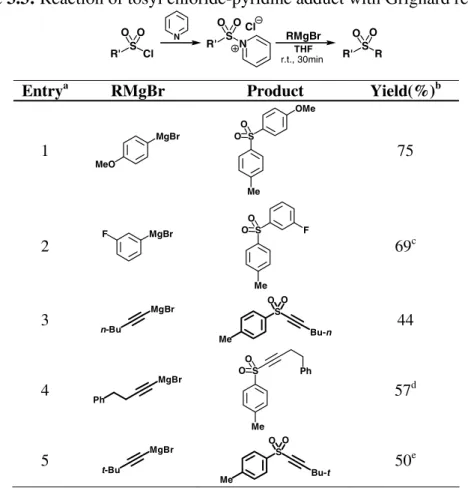
The attention was then directed to other Grignard reagents; alkynyl and other aryl magnesium halides were employed (Table 3.3).
It is interesting to note that this approach is useful in the synthesis of alkynyl and aryl sulfones, but it fails when alkyl Grignard reagents are employed; under these conditions many byproducts are obtained, arising from reduction of the sulfone and S-S coupling, thus affording the sulfone in very low yields (5-10%). Despite this limitation, the reaction can be synthetically useful, because it allows a simple route to alkynyl sulfones, which are obtained in 44-57% yields. These unexceptional yields are however interesting in view of the simplicity of the method used. Alkynyl sulfones can be transformed into cis or trans alkenyl sulfones by means of simple reduction,69 as already discussed (Section 1.2.1).
Table 3.3: Reaction of tosyl chloride-pyridine adduct with Grignard reagents R' S Cl O O R' S R O O N R' S N O O Cl THF RMgBr r.t., 30min
Entrya RMgBr Product Yield(%)b
1 MgBr MeO S O O OMe Me 75 2 F MgBr S O O Me F 69c 3 n-Bu MgBr S Bu-n O O Me 44 4 Ph MgBr S O O Me Ph 57d 5 t-Bu MgBr S Bu-t O O Me 50e a All reactions were performed by quickly adding the Grignard reagent to a
THF solution of tosyl chloride-pyridine adduct at r.t.; b Calculated on the
isolated, chemically pure product; c Conversion was 82%; d Conversion
was 91%; e Conversion was 88%
3.3 Alk-1-enyl sulfones starting from
pyridine-complexed alanes and sulfonyl chlorides in the
presence of Ph
3PO
In the introduction to this chapter, it was pointed out that there were two possible pathways to sulfones, presumably involving intermediates 30 and 31 (Scheme 3.1); with sulfonyl chloride-pyridine adducts, the reaction was described in detail in the previous section. It was, however, unlikely that this intermediate could form a sulfone as byproduct in the sulfoxide formation depicted in Scheme 2.7. It was in fact explained in this chapter that pyridine-complexed alanes fail to
+ (i-Bu)2Al Bu-n 1) Pyridine (20 equiv.) 2) TsCl, PPh3O (15%) CH2Cl2, rfx. Me S O O Bu-n Me S S Me O O 75% 10% 19 21 + (i-Bu)2Al Cl N N
Scheme 3.5: Reaction between pyridine-complexed di-i-butyl hex-1-enyl aluminum with tosyl chloride in the presence of excess pyridine
On the basis of the mechanistic investigation carried out in Section 2.3.3, it is reasonable to assume that, if intermediate 27 afforded sulfoxides, a similar reaction could take place for its analogous 31. The possibility of intermediate 31 was supported by recent literature reports191 which suggest that sulfonyl chlorides react with Ph3PO in pyridine, to afford the corresponding triphenylphosphine
oxide adduct. In order to evaluate a possible catalytic role of triphenylphosphine oxide in the formation of sulfones, a reaction was thus performed using pyridine-complexed di-i-butyl hex-1-enyl aluminum in the presence of 5% of triphenylphosphine oxide. The sulfone was isolated in 48% yield, with a conversion of 51%. The increase of the reaction temperature did not improve significantly the yield, whereas the use of a large excess (20 molar equivalents) of pyridine and of 15% of triphenylphosphine oxide, did allow for quantitative convertion of the sulfonyl chloride into sulfone 19, which was isolated in a 75% yield. The major side product was S-(p-tolyl)-toluene thio sulfonate 21 (Scheme 3.5).
It must be noted that a large excess of pyridine was necessary to achieve good yields of sulfones. In fact, low yields (8-12%) of the sulfone and low conversions (20-25%) were obtained when smaller amounts (two or three equivalents) of pyridine were employed. This behavior is understandable considering that, as already stated, pyridine-complexed alanes are completely unreactive toward unactivated sulfonyl chlorides. Moreover, formation of tosyl chloride-triphenylphosphine adducts occurs only when an excess of pyridine is present.
(i-Bu)2Al R N (i-Bu)2Al R 1) Py (excess) 2) ArSO2Cl Ar S N O O Ph3PO Cl + (i-Bu)2Al R N Ar S O O O Cl + PPh3 S R Ar O O + Ph3PO + (i-Bu)2Al 30 31 N Cl
Scheme 3.6: Reaction between pyridine-complexed di-i-butyl alk-1-enyl alanes with sulfonyl chloride in the presence of excess of pyridine and triphenylphosphine oxide
When insufficient amounts of pyridine are present, all the organometallic reagent is complexed but much of the sulfonyl chloride remains unactivated, and low yields are obtained. When a large excess of pyridine is added, only complexed alane is present in the reaction mixture; in this case triphenylphosphine oxide substitutes pyridine in compound 30, forming intermediate 31. The reasonable assumption that direct displacement of chloride by phosphine oxide is kinetically difficult was verified by means of NMR analysis: 31P spectra did not show any change in the chemical shift of triphenylphosphine oxide upon mixing with tosyl chloride-pyridine complex. The whole process is depicted in Scheme 3.6.
The Ph3PO-catalyzed synthesis of sulfones represented a valuable approach to
these compounds, and its applicability to the preparation of structurally different derivatives was investigated. Results obtained with a few representative organoaluminum compounds are listed in Table 3.4. The nature of the alkyl chain in β position to the aluminum in the organometallic reagent has no influence on reactivity; this is remarkably different with what observed in the previously reported synthesis of sulfones (Section 3.2), and makes this protocol suitable for the synthesis of structurally diverse derivatives.
Entry 7 in Table 3.4 suggests that this protocol is suitable for the synthesis of aryl alkyl sulfones, although the presence of Lewis-acid salts (MgCl2) greatly
this is likely due to the already mentioned reactivity the hydrogen atoms in α position to sulfur atom towards bases such as pyridine.
Table 3.4: Alk-1-enyl sulfones starting from pyridine-complexed alanes and
sulfonyl chlorides in the presence of Ph3PO
R' S Cl O O (i-Bu)2Al R N + Ph3PO R' S R CH2Cl2 1h, rfx. O O + (i-Bu)2Al N Cl Entrya (i-Bu)
2AlR R’SO2Cl Product Yield(%)b
1 Hex-n S Cl O O OOS Hex-n 75 2 Hex-c S Cl O O Me S Me Hex-c O O 70 3 Bu-t S Cl O O Me S Me Bu-t O O 76 4 Bu-n S Cl O O S Ph Bu-n O O 71 5 Ph S Cl O O Me S Me Ph O O 70 6 Bu-n Me S Cl O O Me S Bu-n O O 20 7c Me Me SCl O O Me S Me Me O O Me 63d a All reactions were performed at reflux in CH
2Cl2 using a 20/1/0.15
pyridine/alane/Ph3PO ratio; b Calculated on the isolated, chemically pure
product; c The organometallic reagent was prepared in situ starting from AlCl 3
and (R,S)-2-methylbutyl magnesium chloride; d Maximum yield was obtained
after 92 hours.
In conclusion, this protocol allows one to synthesize aryl alkenyl sulfones in good yields (70-76%), using a one-pot procedure; unlike the previously described
position β to the aluminum atom. This feature makes the presented reaction quite suitable for the synthesis of a wide range of products using a straightforward experimental procedure.