1
GLOSSARY
5-HT: 5-hydroxytryptophan ADA: adenosine deaminase ADP adenosine diphosphate
AICAR: 5-aminoimidazole-4-carboxamide ribonucleotide AK: adenosine kinase
AMP: adenosine monophosphate
AMPK: adenosine monophosphate-activated protein kinase ANOVA: one-way analysis of variance
APCs: antigen-presenting cells
APP: 4-amine-2-(2-hydroxy-1-decyl)pyrazole[3,4-d]pyrimidine ATP: adenosine triphosphate
cAMP: cyclic adenosine monophosphate CD: crohn‟s disease
CD26: adenosine deaminase complexing protein 2 CD39: ecto-apyrase CD73: ecto-5′-nucleotidase
cGMP: cyclic guanosine monophosphate CNS: central nervous system
CNTs: concentrative nucleoside transporters COPD: chronic obstructive pulmonary disease DAG: diacylglycerol
DC: dendritic cells
DHE: dihydroethidium
DNBS: 2,4-dinitrobenzenesulfonic acid DOPA: 3,4-dihydroxyphenylalanine Ecto-ADA: ecto-adenosine deaminase EDTA: ethylenediaminetetraacetic acid
EHNA: erythro-9-(2-hydroxy-3- nonyl)adenine Endo-5′-N: endo-5′-nucleotidase
Endo-ADA: endo-adenosine deaminase ENTs: equilibrative nucleoside transporters ERK1/2: extracellular regulated kinase ½ GABA: γ-amino butyric acid
GBD: glycogen-binding domain GFR: glomerular filtration rate GPCRs: G protein-coupled receptors GTP: guanosine-5‟-triphosphate
2
IBDs: inflammatory bowel diseases
IC50:half maximal inhibitory concentrations ICAM-1: intercellular adhesion molecule-1 IFN-: interferon-
IFN-γ: interferon-γ IL-1: interleukin-1 IL-10: interleukin-10 IL-12: interleukin-12 IP3: inositol triphosphate
Ki: binding affinity of the inhibitor LPS: lipopolysaccharide
MDA: malondialdehyde
MIP-1α: macrophage inflammatory protein-α
MPO: myeloperoxidase
NANC: nonadrenergic noncholinergic
NBTI: nitrobenzylthioinosine NF-kB: nuclear factor-kB NO: nitric oxide
NOD2: nucleotide oligomerization domain 2 NT: nucleoside transporters
NT: nucleoside transporters PBS: phosphate buffered saline PKA: protein kinase A
RIPA: radioimmunoprecipitation assay ROS: reactive oxygen species
SAH hydrolase: S-adenosylhomocysteine hydrolase SAH: S-adenosylhomocysteine
SAM: S-adenosylmethionine SBP: systolic blood pressure
SCID: severe combined immunodeficiency disease
SDS-PAGE: sodium dodecyl sulphate-polyacrylamide gel
electrophoresis Th: T-helper
TNF-α: tumor necrosis factor-α UC: ulcerative colitis
VEGF: vascular endothelial growth factor
ZMP: 5-aminoimidazole-4-carboxamide-1-b-D-ribofuranosyl Monophosphate; AICAr monophosphate
3
4
ADENOSINE: SYNTHESIS AND METABOLISM
Adenosine is an endogenous purine nucleoside released from various tissues and organs and involved in the modulation of a variety of cellular homeostatic mechanisms (synthesis of nucleic acids, metabolism of amino acids and regulation of cellular metabolism) (Noji et al., 2004). This autacoid is also related both structurally and metabolically to the bioactive nucleotides adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP) and cyclic adenosine monophosphate (cAMP) (Poulsen and Quinn, 1998).
The ability of adenosine to regulate several biological functions is strictly related to its extracellular concentration. The levels of this autacoid at its receptors are determined by a variety of mechanisms, which include intracellular and extracellular adenosine biosynthesis, cellular adenosine release, reuptake and metabolism (Noji et al., 2004). These processes are intertwined, subjected to highly dynamic regulation and linked in a complex manner to the energy balance of tissues (Deussen, 2000).
Under physiological conditions, adenosine is formed mainly at the
intracellular level from S-adenosylhomocysteine by
S-adenosylhomocysteine hydrolase, and transported across cell membranes by nucleoside transporters, which play a key role in the control of extracellular adenosine concentrations (Cabrita et al., 2002). After
5
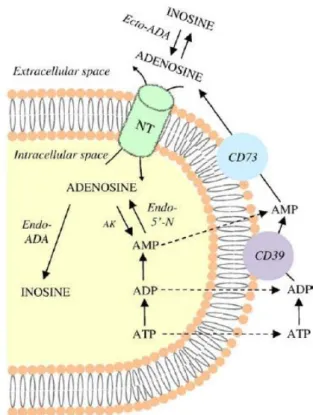
intracellular reuptake, adenosine undergoes rapid phosphorylation to AMP by adenosine kinase, or deamination to inosine by adenosine deaminase. These pathways ensure the maintenance of low intracellular adenosine concentrations through a tight enzymatic control (Noji et al., 2004) (Figure 1A).
Adverse conditions, including hypoxia or inflammation, are associated with increased intracellular and extracellular dephosphorylation of ATP to adenosine through the activity of ecto-apyrase (also named CD39) and 5′- nucleotidase enzymes (endo-5′-nucleotidase and ecto-5′-nucleotidase, also named CD73) in parallel with the suppression of adenosine kinase activity (Deussen, 2000) (Figure 1B). These increments of adenosine levels are aimed at readjustments of the energy supply-to-demand ratio in the pathological tissue via augmentation of local blood flow by vasodilatation, and by down-regulation of the immune response (Niemelä et al., 2004; Sitkovsky & Ohta, 2005).
6
Figure 1. Schematic diagram illustrating the biosynthesis and catabolism of adenosine
under physiological (A) or pathological conditions (B). SAH: S-adenosylhomocysteine; SAH hydrolase: S-adenosylhomocysteine hydrolase; SAM: S-adenosylmethionine; NT: nucleoside transporters; AK: adenosine kinase; ADA: adenosine deaminase; AMP: adenosine monophosphate; ADP: adenosine diphosphate; ATP: adenosine triphosphate; CD39: ectoz-apyrase; CD73: ecto-5′-nucleotidase; Endo-5′-N: endo-5′-nucleotidase; Ecto-ADA: ecto-adenosine deaminase; Endo-ADA: endo-adenosine deaminase (Antonioli et al., 2008b).
ADENOSINE TRANSPORT
Adenosine is transported across cell membranes by nucleoside transporters (NT) which modulate its extracellular concentration and thus its biological actions (Cass et al., 1999; Cabrita et al., 2002). These transporters are classified into two categories according to their molecular and functional characteristics (Noji et al., 2004): 1) equilibrative
7
nucleoside transporters (ENTs); 2) concentrative nucleoside transporters (CNTs) (Gray et al., 2004). Both nucleoside transporters are characterized by broad substrate specificity, low affinity, and high capacity, but are differentiated from each other by their sensitivity to nitrobenzylthioinosine (NBTI).
In particular, equilibrative nucleoside transporters carry nucleosides across cell membranes in either direction, depending on the adenosine concentration gradients across cellular membrane. Based on their sensitivity to nitrobenzylthioinosine (NBTI), this class of nucleoside transport system has been subdivided in two general types: sensitive (es) or
insensitive (ei) transport (Yao et al., 1997; Hyde et al., 2001). The es
transport system is highly sensitive to inhibition by NBTI, while the ei transport system is resistant to this pharmacological blocker (Buolamwini, 1997). At present, four equilibrative transporters (ENT1, ENT2, ENT3 and ENT4) have been identified and cloned (Figure 2). ENT1 and ENT3 are sensitive to NBTI, while this compound does not inhibit ENT2. ENT4 has been identified recently, and its characteristics have not been fully determined. These four members of the equilibrative transport system are widely distributed among various cell types (Podgorska et al., 2005).
8
Figure 2. Diagram of the equilibrative nucleoside transporters (Baldwin et al., 2004).
Concentrative nucleoside transporters promote the intracellular influx of nucleosides against their concentration gradient, using sodium ion gradient across cellular membrane as a source of energy (Gray et al., 2004). These Na+-dependent nucleoside transporters are mainly expressed in the intestinal and renal epithelia, as well as in the liver. They are subdivided in three different subtypes (CNT1, CNT2 and CNT3), depending on their selectivity towards different substrates (Wang et al., 1997; Ritzel et al., 2001) (Figure 3).
9
CNT1 binds pyrimidine nucleosides, but it can also interact with purines. This transporter represents a target for antiviral drugs (zidovudine, lamivudine) or drugs endowed with antineoplastic activity (cytarabine, gemcitabine) (Gray et al., 2004). By contrast, CNT2 transports purine nucleosides. The antiviral drugs didanosine and ribavirin are both substrates of human CNT2 and use this transport system to enter the cell and exert their pharmacological activity. CNT3 has been cloned more recently. It transports both purine and pyrimidine nucleosides into cells in a sodium-dependent manner (Ritzel et al., 2001).
Figure 3. Diagram showing the concentrative nucleoside transporters (CNT1, CNT2,
CNT3) (Gray et al., 2004).
Most studies have focused their attention on the role played by es nucleoside transporters since they are widely expressed throughout the
10
body and modulate several physiological functions in many tissues (Noji et
al., 2004).
ADENOSINE METABOLISM
Inactivation of extracellular adenosine signaling is accomplished mainly by its reuptake across cell membrane by nucleoside transporters, followed by either phosphorylation to AMP under physiological conditions by adenosine kinase (AK), critically important in maintaining low levels of adenosine, or deamination to inosine by adenosine deaminase (ADA) (Haskò and Crostein, 2004).
Adenosine deaminase is a 36-kDa, high capacity and high Km enzyme, regarded mainly as a cytosolic enzyme (endo-adenosine deaminase). However, it can be expressed also on the external membrane surface of several immune and non-immune cells (ecto-adenosine deaminase) (Cristalli et al., 2001; Latini & Pedata, 2001). This enzyme has a wide phylogenetic distribution and its amino acid sequence is highly conserved from bacteria to humans suggesting that ADA is a key enzyme in the purine metabolism. This enzyme is present in virtually all human tissues, but the highest levels are found in the lymphoid system inclucing lymph nodes, spleen, and thymus. In this regard, genetic defects in the enzyme cause severe combined immunodeficiency disease (SCID) by impairment
11
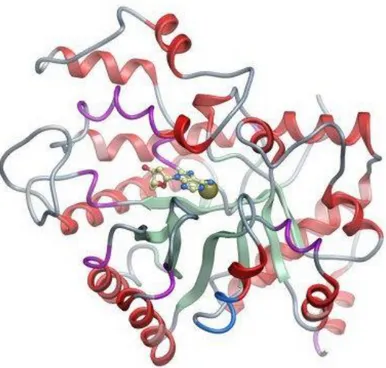
of both T and B cell functions (Cristalli et al., 2001). In 1991, the three-dimensional structure of recombinant murine adenosine deaminase expressed in Escherichia coli was described by Wilson and co-workers (Wilson et al., 1991). The enzyme complexed with an inhibitor resembling the tetrahedral intermediate (transition-state analog) contains a parallel α/β-barrel motif with eight central β strands and eight peripheral α helices (Figure 4). The oblong-shaped deep active site is lined by the COOH-terminal segments and connecting loops of the β-barrel strands. Interestingly, the enzyme contains a zinc atom which participates directly in the deamination mechanism (Figure 4). The stereospecificity of enzyme/substrate interaction is conferred by the location of zinc and of a histidine and aspartate residue, and it is characterized by several hydrogen bonds that stabilize the binding of substrate and the transition state (Orozco et al., 1990; Wilson and Quiocho, 1993) (Figure 4).
12
Figure 4. Ribbon diagram of human adenosine deaminase enzyme. Purine unit of an
inhibitor lies above the zinc atom visible at center (from ‘thesgc.org’).
Inhibition of ADA has been postulated since many years to be useful in the treatment of different disorders (Smyth et al., 1985; Spiers, 1985; Johnson et al., 1986). The primary advantage of this approach is that the increment of extracellular adenosine levels may be restricted primarily to those sites where endogenous extracellular adenosine formation is greatest, for instance in hypoxic/ischemic tissues, or where an excessive stimulation occurs. In this regard, a close correlation has been found between the severity of inflammation and a local increase in both expression and activity of this enzyme, leading to a decreased availability of biologically active adenosine (Conlon and Law, 2004; Desrosiers et al., 2007). Based on this knowledge, the pharmacological inhibition of adenosine deaminase
13
is being regarded as a novel therapeutic approach to counteract inflammation in several pathological conditions (Adanin et al., 2002; Law et al., 2003; Kayhan et al., 2008, Antonioli et al., 2010b).
INTERACTION BETWEEN AMP-ACTIVATED PROTEIN KINASE (AMPK) AND ADENOSINE SYSTEM
Recent evidence suggests an interaction between adenosine monophosphate-activated protein kinase (AMPK) and the adenosine system (Aymerich et al., 2006; Pang et al., 2010). Indeed, the activation of this enzyme results in increments of both AMP and adenosine levels under conditions of energy deprivation (Drew and Kingwell, 2008).
AMPK is an enzymatic complex involved in the regulation of energy balance at both cellular and whole-body levels (Hardie, 2008). It is a heterotrimeric complex comprising a catalytic subunit (α) and two regulatory subunits (β and γ) (Hardie et al., 2003) (Figure 5). Homologues of all three subunits have been identified in every eukaryotic species examined to date, ranging from mammals, fruit fly, worm, yeast and plants. This high conservation degree suggests that formation of the heterotrimeric complex is an essential requirement for at least some functions of this kinase.
14
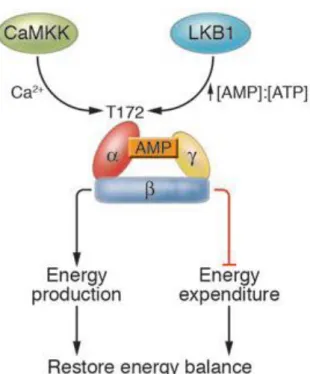
Figure 5. AMPK maintains the intracellular energy balance. Conditions that cause an
increase in the AMP:ATP ratio lead to activation of AMPK through increased phosphorylation on Thr172 within the α subunit. Recent evidence suggests that LKB1 is the upstream kinase in the cascade, although how LKB1 activity is regulated is not yet known (Long and Zierath, 2006).
In mammals, isoforms of all three subunits, which are encoded by separate genes, have been identified. The roles of the different subunits within the AMPK complex provide important clues regarding the physiological functions of the kinase, and valuable insights into its regulation (Carling, 2004). Each subunit exists as alternate isoforms encoded by two or three genes (α1, α2, β1, β2, γ1, γ2, γ3), and all the twelve different combinations of these isoforms appear to be able to form functioning complexes. The predominant isoforms in most cells are α1-β1-γ1, but liver cells significantly express also α2 (Woods et al., 1996), while
15
skeletal and cardiac muscles express also α2, β2, γ2 and γ3 (Stapleton et al., 1996).
Subunit : the N-terminal half of the α subunit contains a typical serine/threonine protein kinase catalytic domain, with features conserved throughout the protein kinase superfamily (Hanks et al., 1988). The α subunit also contains several residues that can be phosphorylated both in vitro and in vivo. One of these residues is Thr172 and its phosphorylation is essential for AMPK activity.
Subunit : it appears that one of the functions of the β subunit is to act as a scaffold for binding the α and γ subunits (Woods et al., 1996). This subunit contains a central conserved domain that has recently been recognized as a glycogen-binding domain (GBD) involved in the interaction of AMPK with glycogen (Figure 6). This non-catalytic domain provides a mechanism for regulation of AMPK by glycogen. However, the physiological meaning of this domain remains unknown (Carling and Hardie, 1989; Wojtaszewski et al., 2002).
Subunit : this subunit contains three AMP-binding sites, located at the interface of its two pairs of CBS domains or Bateman domains. Two of these binding sites can bind either AMP or ATP, while a third site contains a tightly bound AMP that does not exchange (Xiao et al., 2007). When AMPK is inactive under physiological conditions, it binds two ATP and
16
one AMP molecule, while under low energy states it binds three AMP molecules. It has been proposed that the interaction of the catalytically active kinase domain with the AMP-bound γ-subunit protects the phosphorylated Thr172 residue from dephosphorylation (Figure 6).
Figure 6. AMPK (blu) (green) and (red) core structures. Graphical representation of composite features from the mammalian AMPK heterotrimer showing the relative positions of three CBS occupied by AMP, with ADP modelled into site 2 (Steinberg and Kemp, 2009).
As suggested by its name, AMPK is allosterically activated by AMP, but, more importantly, it is also activated by phosphorylation by one or more upstream kinases at the level of a threonine residue within the activation loop of the α-subunit kinase domain (Hardie, 2003). These stimulant effects of AMP are antagonized by high concentrations of ATP, so that the system responds to rises in the AMP:ATP ratio rather than to rises in AMP alone.
17
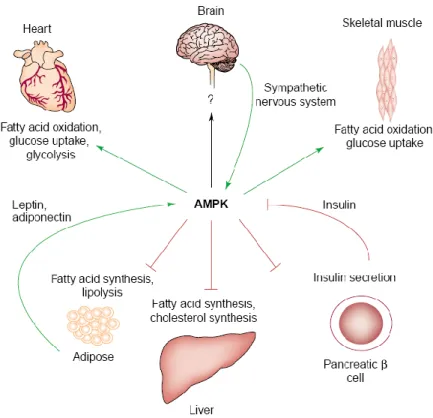
Once activated, AMPK phosphorylates several downstream substrates aimed at switching off ATP-consuming pathways (e.g., fatty acid synthesis, cholesterol synthesis), and switching on ATP-generating pathways (e.g., fatty acid oxidation and glycolysis) (Kemp et al., 1999; Carling, 2004). In addition to the acute effects of AMPK on energy metabolism, the activation of this kinase has long-term effects, altering both gene expression (Yang et al., 2001)and protein expression (Winder et al., 2000) (Figure 7).
Figure 7. Proposed roles of AMP-activated protein kinase (AMPK) in the control of
whole-body energy metabolism. Activation of AMPK stimulates energy-generating pathways (green arrows) in several tissues while inhibits energy-consuming pathways (red lines) (Carling, 2004).
Although the physiological consequences of these long-term effects of AMPK are not fully understood, it seems likely that they are involved in
18
the overall regulation of energy metabolism. The discovery of naturally occurring mutations in AMPK, that cause cardiac hypertrophy, provides direct evidence that AMPK plays a pivotal role in maintaining normal human physiology (Blair et al., 2001; Gollob et al., 2001).
This enzymatic complex is involved in the regulation of energy-demanding/consuming metabolic pathways in several pathophysiological settings including ischemic/inflammatory diseases (Drew and Kingwell, 2008). Recent evidence indicates that AMPKα1 deficient mice develop an exacerbated experimental autoimmune encephalomyelitis disease severity compared with wild type mice (Nath et al., 2009). In this regard, the pharmacological modulation AMPK can be viewed as a promising therapeutical approach for the treatment of inflammatory conditions (Drew and Kingwell, 2008). In particular, AMPK activation can suppress proinflammatory responses in macrophages (Jeong et al., 2009). Accordingly, AMPK activation inhibits lipopolysaccharide (LPS)-induced proinflammatory responses in murine neutrophils and diminished the severity of acute lung injury in mice (Zhao et al., 2008). Moreover, Peairs et al. (2009) showed that the phosphorylation of AMPK markedly inhibits LPS/IFN-γ-stimulated inflammatory mediators in mesangial cells isolated from MRL/MPJ-Faslpr mice.
19
ADENOSINE RECEPTORS
Almost 80% of the endogenous ligands involved in the regulation of cellular functions (hormones and neurotransmitters) exert their effects via interaction with specific G protein-coupled receptors (GPCRs).
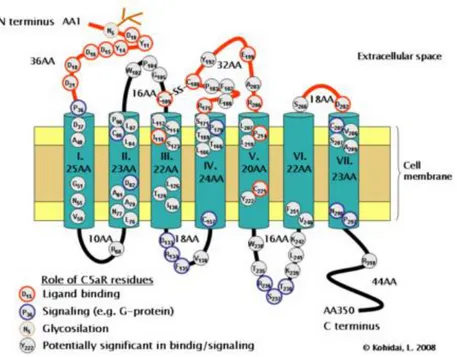
All adenosine receptors are coupled to G proteins and display the common architecture of seven membrane-spanning helices connected by intra- and extracellular loops. The N-terminal of the protein lies on the extracellular side and the C-terminal on the cytoplasmic side of cell membrane. A pocket for the ligand binding site is formed by the three-dimensional arrangement of the α-helical transmembrane domains, and the agonist is believed to bind within the upper half of this pore (Figure 8) (Ralevich e Burnstock, 1998).
Figure 8. Transmembrane topology of a typical G protein-coupled receptor (Kohidai
20
Variable structural characteristics of G protein-coupled receptors are associated with the third cytoplasmic loop length and carboxyl terminal. The interaction between agonist and the receptor binding site causes a conformational change of the receptor, especially in the third cytoplasmic loop and the carboxyl terminal that is transmitted to the G protein. This protein binds to different contact points. The differences in the third cytoplasmic loop structure and carboxylic terminal enable specificity for different receptors to bind with different G proteins. The cytoplasmic part of the receptor, particularly the carboxylic tail, contains severa phosphorylation sites (Figure 8). These sites are functionally relevant since they mediate the uncoupling of the receptor from the G protein, all being responsible for desensibilization (Luttrell, 2006).
The formation of receptor-protein complex represents a crucial step for signal transmission. G proteins are heterotrimers formed by three different subunits: , , and . Through biochemical and molecular studies, the number of known subunits has progressively increased, and nowadays twenty G protein subtypes have been classified, based on their difference in the structural and functional characteristic of the subunit. Beside the 20 subunits identified, five subunits and ten subunits are known. This suggests that or chain may form a heterotrimer with different
21
subunits. The subunits are able to bind guanilyl nucleotides, in particular the guanosine-5‟-triphosphate (GTP), and promote their hydrolization.
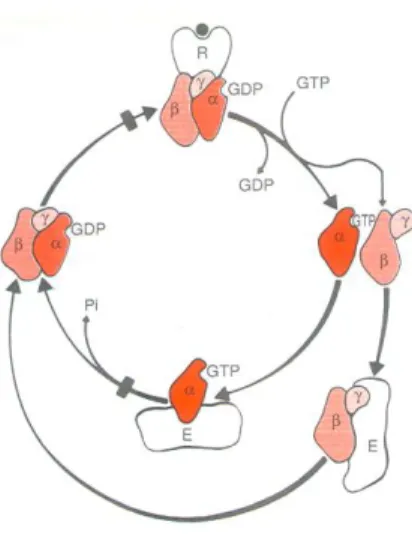
These biochemical activities are crucial for the signal transduction of G-proteins, which are able to transfer stimuli from receptors to the effector molecules through an activation/inactivation cycle regulated by the binding and hydrolysis of GTP (Figure 9) (Clementi e Fumagalli, 1999).
Figure 9. Diagram representing the activation-inactivation cycle of G protein-coupled
receptors (Clementi e Fumagalli, 1999).
In 1978, Burnstock proposed a classification for purinergic receptors into P1 and P2 receptors, based on the relative potencies of nucleoside and nucleotides (ATP, ADP, AMP and adenosine) as well as on the selective actions of antagonists, particularly the methylxanthines. P1 purinoceptors are more responsive to adenosine and methylxanthines (theophylline and caffeine) act as selective competitive antagonists on these receptors. Moreover, the occupation of P1 purinoceptors leads to inhibition or
22
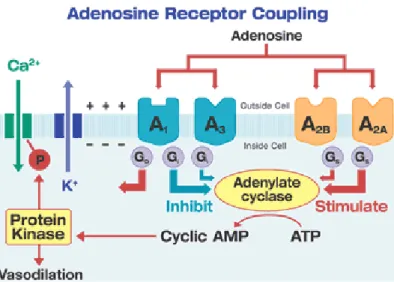
activation of an adenylate cyclase system with consequent changes in intracellular cAMP levels. P1 receptors are further divided into four subtypes, A1, A2A, A2B, and A3, on the basis of their distinct molecular structures, tissues distribution and pharmacological profiles (Fredholm et al., 2001) and, as anticipated above, they all couple G proteins. It is well known that A1 and A3 receptors couple to Gi/o and that A2A and A2B receptors couple to Gs (Figure 10). The stimulation of these receptors can determine the activation or inhibition of adenylyl cyclase. Moreover, in some tissues, A1 and A3 receptors can modulate phospholipase C activity, A1 receptor can also modulate the activity of Ca2+ or K+ channels (Clementi e Fumagalli, 1999).
Figure 10. Representative scheme of adenosine receptor subtypes and their second
23
Adenosine receptor subtypes have also been distinguished on the basis of their high (A1, A2a) or low (A2b, A3) levels of affinity for their endogenous ligand (Poulsen and Quinn, 1998).
A1 receptor. It was firstly identified in the central nervous system and
localized on dopaminergic fibers (Murayama et al., 1990). A1 receptor mediates a broad range of signaling responses though its coupling to Gi or Go proteins. The main intracellular signaling related to A1 receptor activation is the inhibition of adenylate cyclase, followed by a decrease in the levels of the second-messenger cAMP (Van Calker et al., 1979; Londos et al., 1980). It has been demonstrated that A1 receptor activation is linked to hyperpolarization of cell membranes through the activation of potassium current. In addition, the activation of A1 receptors at presynaptic levels inhibits Ca2+ channels with a reduction of neurotransmitter release (Klinger et al., 2002). Studies conducted on human cells expressing A1 receptors, have then showed that the interaction of adenosine with these receptors can activate the protein kinase extracellular regulated kinase 1/2 (ERK1/2) (Fredholm et al., 2001).
A2A receptor. A2A like A2B receptors, have been identified in mast cells
isolated from human bronchoalveolar lavage, thus suggesting an important role in the pathogenesis of asthma and chronic obstructive pulmonary
24
disease (COPD) (Polosa, 2002). A2A receptors are coupled to Gs protein, and this pathway induces the activation of adenylate cyclase (Klinger et al., 2002). The identification and characterization of potent selective A2A receptor ligand has allowed the characterization of different functional responses induced by activation or inhibition of this receptor, and hence the comprehension of its biological relevance in physiological as well as pathological conditions, such as ischemia/hypoxia (Pedata et al., 2005).
A2B receptor. This receptor has been cloned from human hippocampus
(Pierce et al., 1992), rat brain (Rivkees and Reppert, 1992), and mouse bone marrow derived mast cells (Marquardt et al., 1994). Since cells typically respond with an increase in cAMP, the receptor was proposed to represent a Gs-coupled receptor (Fredholm et al., 2001). This receptor can also couple Gq protein, causing an increment in IP3 levels and reverse of calcium ions from cell depots (Klinger et al., 2002).
A3 receptor. A3 receptors were identified in the rat kidney, cardiac
tissue, brain, lungs and testis. In humans, A3 receptors are present in the liver, placenta, kidneys, brain, aorta, heart, lungs, testis and gastrointestinal tract (Fredholm et al., 2001).
This receptor subtype has the same transduction mechanism of A1 receptors, where G protein is inhibitory and can thus reduce the adenylate
25
cyclase activity. However, it has been shown that the activation of A3 receptor may also result in an increment of IP3 levels, with subsequent activation of calcium channels (Klinger et al., 2002).
P2 purinoceptors are more responsive to ATP and ADP than adenosine, moreover, these receptors are not antagonized by methylxanthines and they do not act via an adenylate cyclase system (Burnstock, 1978). P2 receptors include ligand-gated cation channels (Benham and Tsien, 1987) or G protein coupled receptors (Dubyak, 1991). Based on these features, they have been subdivided into two main groups, designated as P2X receptors (ligand-gated cation channels) and P2Y receptors (G protein-coupled receptors) (Abbracchio and Burnstock, 1994).
26
BIOLOGICAL FUNCTIONS OF ADENOSINE
In 1970, Burnstock proposed the novel concept that adenosine triphosphate (ATP) and related nucleotides/nucleosides (adenosine diphosphate, ADP; adenosine monophosphate, AMP; adenosine) act as transmitters involved in nonadrenergic noncholinergic (NANC)-mediated relaxing responses of smooth muscle in the gastrointestinal tract and bladder. Two years later, the term “purinergic” was coined and the purinergic neurotransmission hypothesis was put forward (Burnstock, 1972). This concept initially met considerable resistance and skepticism in the scientific community, used to regard the purine system only as a ubiquitous biochemical source of energy, but subsequently it met wide acceptance and became a cornerstone of several systems.
Adenosine is now recognized as an important regulator of neurotransmission in the central nervous system (CNS), where it is involved in the modulation of several physiological functions, such as regulation of arousal and sleep, anxiety, cognition and memory (Ribeiro et al., 2003). In the CNS, adenosine is available at low concentrations in the extracellular fluids, and exerts inhibitory effects on different neuronal populations through the activation of A1 receptors (Ribiero, 2005), reducing the presynaptic release of different neurotransmitters (Abbracchio and Cattabeni, 1999; Stone and Bartrup 2002), and enhancing
27
potassium conductance at post-synaptic level (Stone and Bartrup, 2002). Experimental evidence has demonstrated the capacity of A1 receptor to inhibit the release of different neurotransmitters, such as dopamine (Cauli and Morelli), serotonin, noradrenaline, acetylcholine (Sawynok and Liu, 2003), and glutamate (Ribeiro, 2005). On the other hand, stimulation of A2A receptors has been associated with facilitation of the dopaminergic and cholinergic transmission (Rosin et al., 1998) as well as with inhibition of GABA-ergic transmission (Moreau and Huber, 1999).
Some authors have hypothesized a possible neuroprotective role for adenosine in the central nervous system, based on its capacity to prevent the damage associated with ischemic events or epileptic attack, where adenosine levels increase significantly, through the modulation of excitatory and inhibitory amino acid release (Pagonopoulou et al., 2006; Yildirim et al., 2007). In spite of the commonly accepted neuroprotective role of adenosine, recent studies suggest that this nucleoside could unexpectedly, under certain circumstances, exert opposite effects contributing to the neuronal damage and death. The basis for this duality may rely on the activation of different adenosine receptors subtypes, and the roles played by these receptors on different functions of neuronal and glial cells (de Mendonca et al., 2000). Current knowledge of the roles played by adenosine and its analogues at the level of CNS suggests
28
therapeutic implications of purinergic antagonists for treatment of neurodegenerative diseases such as Parkinson‟s disease, Alzheimer‟s disease and Huntington‟s disease (Klinger et al., 2002; Schwarzschild et al., 2006). In this regard, some authors have proposed A1 receptor antagonists as a strategy for treatment of Alzheimer disease, since they promote cholinergic transmission through the enhancement of acetylcholine release (Sebastião and Ribeiro, 1996). Moreover, A2A antagonists have a great potential in the treatment of Parkinson‟s disease and are now entering clinical trials (Schwarzschild et al., 2002). In particular, they not only reduce Parkinsonian-like muscle rigidity in rats, but potentiate also the effect of l-DOPA, which may allow the use of lower doses of this drug (Wardas et al., 2001).
Recent progress has been made mainly with regard for the role of adenosine as cardioprotective agent. At the cardiac level, adenosine is implicated in the regulation of pacemaker cells and cardiomiocytes (Mubagwa and Flameng, 2001). This autacoid exerts a negative inotropic and cronotropic effect, ascribed to its interaction with cardiomyocyte A1 receptors, that counteract the positive inotropic and cronotropic effects of catecholamines. In particular, in the atrium, adenosine evokes K+ conductance, while in the ventricles, the same effects are attributed to the reduction of Ca2+ influx. Nowadays, the activity of purines at the cardiac
29
level is therapeutically useful for management of supraventricular arrhythmias. Moreover, several experimental observations have shown that this nucleoside act as a potent vasodilator, which plays a major role in the control of coronary blood flow during metabolic stress, and has particular implication in ischemic preconditioning (Ely and Berne, 1992) or hypoxia (Nakhostine and Lamontagne, 1993). In particular, adenosine causes a reduction in cardiac work and myocardial oxygen consumption through a decrease in cardiac rate, inhibition of senoatrial node conductance by A1 receptors, and subsequent activation of ATP-dependent potassium channels. Adenosine is also implicated in the control of coronary flux (Hein and Kuo, 1999). Indeed, it causes vasodilatation through the activation of A2A and A2B receptors, expressed mainly on coronary vessels (Poulsen et al., 1998). A2A activation also contributes significantly to basal nitric oxide (NO) production, increasing cGMP levels and thus favoring the dissociation of myosin and actin filaments, responsible for the contractile response (Mubagwa and Flameng, 2001). On the same line, several authors have described an anti-ischemic effect of adenosine mediated by A2A receptor (Forman et al., 2006). This effect seems to be mediated by two mechanisms: the first leads to activation of the receptor and consequent increment of cAMP production. This triggers a phosphorylative cascade of ERK proteins and subsequent activation of
30
Rap1 protein, which favors a prolonged cellular survive during hypoxia (Forman et al., 2006). The second mechanism hypothesizes the reactivation of K+ and Ca2+ influx currents, with the activation of PKA (phosphorylative cascade), enabling a protective action towards the ischemic condition (Klinger et al., 2002). Adenosine, through A2A and A2B receptors, exerts also a potent inhibitory effect on platelet aggregation (Horiuchi, 2006). This autacoid can thus reduce myocardial damage, attenuate reversible post-ischemic ventricular dysfunction and reduce the severity of myocardial infarct (Liang e Jacobson, 1998; Baxter, 2002).
At level of the respiratory system, adenosine plays a key role in inflammatory processes and bronchoconstriction associated with asthma or chronic obstructive pulmonary disease (COPD). Inhalation of this purine, usually administered as AMP, initiates narrowing of airways in asthmatic patients or allergic non-asthmatic subjects (Fan and Mustafa, 2002). Investigations into the mechanism of action of adenosine-induced bronchoconstriction suggest that it is unlikely that this autacoid acts directly on smooth muscle cells, but rather indirectly through the activation of purinergic receptors expressed on intermediary inflammatory cells, such as airway mast cells or afferent nerve endings. This action results in the prompt release of contractile inflammatory mediators, such as leukotrienes, prostaglandins, interleukins, tumor necrosis factor-α
31
(TNF-α) and histamine. In this regard, experimental evidence has demonstrated that adenosine acts through two mechanisms in the induction of broncho-alveolar constriction: neuronal mechanism and the interaction with purinergic receptors on mastocyte membrane (Spicuzza et al., 2003). Moreover, in vitro studies have shown that adenosine can induce the liberation of histamine, prostaglandin D2 and tryptase from mast cells (Spicuzza et al., 2003). Adenosine receptors expressed on these cells belong to A2A and A2B subtypes. Under physiological conditions, the low concentration of adenosine is enough to activate high affinity A2A receptors, while in the presence of high adenosine concentrations, such as in asthma and COPD, low affinity A2B receptors are also enrolled, causing degranulation of mast cell by IP3 and DAG, and the increase in histamine release (Spicuzza et al., 2003).
At the renal level, adenosine exerts its functions through interaction with A1 and A2 receptor subtypes. Contrast to what happens in the heart and extra-renal vessels, where the nucleoside exerts vasodilatory effects, in the kidney it exerts mainly a vasoconstrictive effect. Indeed, both adenosine and purinergic agonists can reduce renal blood flow with the concomitant reduction of glomerular filtration rate (GFR). These effects are mediated by A1 receptors located on afferent arterioles, at the glomerular level, in the proximal tubule and collecting ducts (Welch,
32
2002). A2A receptors are expressed mainly on efferent arterioles (Modlinger e Welch, 2003).
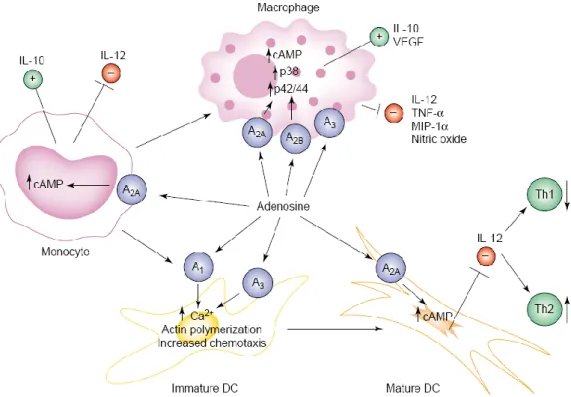
Several lines of evidence have shown that adenosine plays prominent roles in maintaining tissue integrity by modulation of immune functions, downregulation of phlogistic reactions, interference with biosynthesis of proinflammatory cytokines, inhibition of neutrophil adhesion, degranulation, and oxidant activity (Antonioli et al., 2008a). In particular, detailed studies have indicated adenosine as a prominent player deputed to down-regulate activated immune cells and protect tissues from inflammatory injury via receptors expressed on immune cell populations (lymphocytes, neutrophils, monocytes, macrophages, dendritic cells and mast cells) (Haskò & Cronstein, 2004; Haskò et al., 2007) (Figure 11).
33
Figure 11. Scheme rapresenting the modulatory role of adenosine on different immune
cell populations. DC: dendritic cells; IL-10: interleukin-10; IL-12: interleukin-12; VEGF: vascular endothelial growth factor; TNF-α: tumor necrosis factor-α; MIP-1α: macrophage inflammatory protein-α (Haskò and Cronstein, 2004).
Cytotoxic T lymphocytes and T-helper (Th) cells are endowed with a marked expression of A2A, A2B and A3 receptors, whereas A1 receptors are scarce or absent in these cells (Lukashev et al., 2003; Gessi et al., 2005). A significant up-regulation of A2B receptors has been observed following activation of human peripheral CD4+ and CD8+ T cells (Lappas et al., 2005). Analogously, evidence of a rapid up-regulation has been reported by Gessi et al. (2004) for A3 receptors upon activation of human T lymphocytes. Recent findings have shown a dual opposite action of adenosine on T cell proliferation through A2A (stimulatory) and A3
34
(inhibitory) receptors (Takahashi et al., 2007). However, the scarcity of data regarding the expression and function of A3 receptors in lymphocytes warrants additional investigations, particularly to verify whether experimental findings can be translated to the human gut immune system.
Adenosine can suppress the expression of intercellular adhesion molecule-1 (ICAM-1) in lymphocytes from enteric Peyer‟s patches (Johnston et al., 2005). Based on this mechanism, extracellular adenosine may inhibit ongoing accumulation of lymphocytes at inflammatory sites by limiting their adhesion and migration into extravascular sites (Yang et al., 2005). Adenosine receptors appear to be involved also in cytokine production in activated Th cells. Indeed, A2A receptors are predominantly expressed in human cytokine-producing T cells and the stimulation of Th cells with anti-CD3 monoclonal antibodies was shown to evoke a rapid up-regulation of these receptors, with subsequent decrease in the release of interferon- (IFN-) (Lappas et al., 2005). More recently, Deaglio et al. (2007) investigated the role played by CD39 and CD73 in the control of lymphocyte activity. Their results indicate that the co-expression of these enzymes on cell surface distinguishes CD4+, CD25+ and Foxp3+ T regulatory cells from other T cells, suggesting that both enzymes are suitable cell surface markers. Moreover, Deaglio et al. (2007) have shown that these ecto-enzymes convert extracellular nucleotides into pericellular
35
adenosine, which then induces immune suppression through stimulation of A2A receptors on activated T effector cells, indicating a relevant role of CD39 and CD73 in the modulating functions of T regulatory cells.
Adenosine is abundantly released from neutrophils following their activation, and contributes actively to the regulation of these cells during inflammatory responses (Linden, 2006). Neutrophils express both A1 and A2A receptors, and their functions appear to be modulated by variations in extracellular adenosine levels. In particular, stimulation of A1 receptors induces up-regulation of the neutrophil adhesion receptor Mac-1 and increased expression of complement receptors responsible for enhanced adhesion of neutrophils to vascular endothelium (Bours et al., 2006). By contrast, the activation of A2A and A2B receptors, via adenosine produced by CD39 and CD73 during inflammation, inhibits the adhesion of neutrophils to endothelial cells (Eltzschig et al., 2004). Molecular studies have demonstrated the expression of A3 receptors on human neutrophils, but their functional characterization is still lacking (Gessi et al., 2002). Adenosine exerts a protective action on host tissues through modulation of neutrophil bactericidal functions. A dual regulatory influence of adenosine on phagocytosis has been reported, since the activation of A1 receptors enhances this process, while the stimulation of A2A receptors causes a marked reduction of phagocytic activity (Zalavary & Bengtsson, 1998).
36
There is also evidence that adenosine can differentially regulate the generation of reactive oxygen species (ROS) with stimulatory or inhibitory actions depending on its predominant levels at different receptor sites. Indeed, adenosine promotes the production of ROS from activated neutrophils through recruitment of A1 receptors, whereas it down-regulates ROS generation via A2A receptors (Sun et al., 2007). Moreover, adenosine exerts a strict control over the release of microbicidal agents from neutrophils via interaction with A2A and A3 receptors (Theron et al., 2002).
Monocytes and macrophages express all four adenosine receptors, and recent studies have demonstrated significant variations in their expression and function during the maturation process: the expression of A1, A2A and A3 receptors appears to be low in quiescent monocytes, while their density increases during differentiation into active macrophages (Thiele et al., 2004). The patterns of purinergic receptor expression are sensitive to the release of cytokines in response to inflammatory stimuli. In particular, A2A receptors undergo up-regulation in human monocytes stimulated by interleukin-1 (IL-1) and tumor necrosis factor- (TNF-), while treatment with IFN- significantly reduces their expression (Khoa et al., 2001). A regulatory role of adenosine in the recruitment of monocytes at inflammation sites, through A2A and A2B receptor-mediated inhibition of
37
cell adhesion molecule expression on endothelium, has been also described (Delikouras et al., 2003).
Several studies have investigated the effects of adenosine on cytokine production in monocytes and macrophages. Current evidence indicates that production of IL-12, TNF-, IL-6, macrophage inflammatory protein (MIP)-1 and nitric oxide (NO) is negatively affected by adenosine through activation of A2A, A2B and A3 receptors (Haskò et al., 2000; Ohta and Sitkovsky, 2001; Haskò et al., 2007). In addition, extracellular adenosine stimulates the release of the anti-inflammatory cytokine IL-10 by monocytes and macrophages via A2A and A2B receptors, thus promoting the termination of inflammatory responses (Khoa et al., 2001; Nèmeth et al., 2005).
Dendritic cells belong to a specialized system of antigen-presenting cells (APCs), which play an important role in the stimulation of the immune response, participating in antigen interception, processing, and inducing the activation of specific lymphocyte-effector mechanisms (Haskò and Cronstein, 2004). Adenosine has been shown to modulate dendritic cell functions by interaction with specific receptors, the expression and function of which is strictly related to the maturation status of these immune cells (Haskò and Cronstein, 2004). Immature human dendritic cells express predominantly A1 and A3 receptors, which mediate
38
chemotaxis via an increase in intracellular calcium, thus allowing the recruitment of dendritic cells into inflammation sites. Activated mature dendritic cells are endowed mainly with A2A receptors, which confer sensitivity to the anti-inflammatory effects of adenosine, with a significant reduction of cytokine production and compensatory effects against chronic cell activation, responsible for tissue damage (Schnurr et al., 2004). Moreover, Pacheco et al. (2005) have demonstrated the presence of A2B receptors in both immature and mature dendritic cells, where they act as adenosine deaminase anchoring proteins. Adenosine deaminase and A2B receptor form a molecular complex that interacts with CD26 expressed on T cells and evokes a co-stimulatory signal on IFN- and TNF- production. Dickenson et al. (2003) performed studies on the murine dendritic cell line XS-106, demonstrating that A2A and A3 receptors inhibit the release of TNF-α, and proposed these cells as a useful model to evaluate the role of adenosine in the regulation of dendritic cell functions. Since most investigations, designed to examine the effects of adenosine on APC functions, have been performed on single cell lines or a single source of APCs, additional studies, conducted on different cell models, might help to better determine the role played by adenosine in these immune cells.
39
Mast cells are generally recognized as crucial players in allergic reactions and important initiators of innate immune responses. They can also have a role in the pathogenesis of inflammatory processes and pain sensation (Thacker et al., 2007). Interestingly, mast cells might be the exception to the general „rule‟, according to which adenosine promotes immunosuppression and tissue protection, since this nucleoside acts as a potent stimulator of mast-cell function. In particular, adenosine, mainly through occupancy of A2B and A3 receptors, stimulates the degranulation of mast cells, which then release histamine, 5-HT, chemokines and proteases (Haskò and Cronstein, 2004). There is also evidence that histamine, released from mast cells in response to adenosine, elicits inhibitory effects, via interaction with macrophage H2 histamine receptors, on TNF- biosynthesis (Smith et al., 2002). Nevertheless, the proinflammatory actions resulting from direct adenosine-induced mast cell stimulation seem to overcome the anti-inflammatory response promoted by adenosine via the histamine-mediated negative feedback on mast cell activation (Haskò and Cronstein, 2004).
40
EFFECTS OF ADENOSINE ON GASTROINTESTINAL FUNCTIONS There is evidence supporting the involvement of adenosine in the regulation of physiological functions in the gastrointestinal tract (Antonioli et al., 2008b). Adenosine has been shown to modulate the release of gastric regulatory peptides (Kwok et al., 1990). In particular A1 and A2A receptor expression was found were shown to be expressed throughout the antral and corporeal mucosa of stomach (Yip et al., 2004). Subsequently,
in vivo experiment conducted on rat showed that adenosine inhibits gastrin
release through interaction with A1 receptor while it stimulates somatostatin release via interaction with A2A receptors (Yip and Kwok, 2004).
At level of the normal digestive tract, adenosine contributes to the control of enteric neurotransmission and smooth muscle contractility, thus participating to the physiological regulation of gut motor functions through interaction with specific receptors located on enteric neurons at pre- and post-junctional levels (Christofi et al., 2001; Kadowaki et al., 2003). In vitro studies, conducted on rodent small intestine, demonstrated that
adenosine modulates acetylcholine and substance P release through inhibitory pre-junctional A1 receptors on enteric neurons (Nicholls and
Hourani, 1997; Kadowaki et al., 2003). These findings support previous in
41
markedly reduced gut propulsion in rat small bowel, while an A1
antagonist increased significantly the peristaltic activity (Kadowaki et al., 2000). By contrast, Duarte-Araùjo et al. (2004) have demonstrated an A2A
receptor-mediated facilitatory effect on acetylcholine release in rat ileum. Moreover, recent findings suggested the involvement of A2A receptors in
the inhibitory control of rat colonic motility via recruitment of inhibitory nitrergic mechanisms (Antonioli et al., 2006). A2B receptors are located on
smooth muscle cells and their stimulation results in a marked inhibition of peristaltic activity both in the distal colon (Nicholls et al., 1996) and rat duodenum (Bailey e Hourani, 1992, Fozard et al., 2003).
At intestinal level, adenosine seems to be implicated also in the regulation of secretory and reabsorbative processes through A2B receptors expressed on immune cell population of intestinal mucosa (Nicholls et al., 1996). A2A receptors may contribute to the development of diarrhea.
Adenosine acts therefore as a paracrine mediator which regulates the secretion of Cl− ions (Madara et al., 1993).
By means of immunohistochemical analysis, a heterogeneous distribution of all four adenosine receptors (A1, A2A, A2B, A3) has been observed in different regions of human gut. However, further studies are warranted to elucidate the differential role of adenosine receptor subtypes
42
in the control of absorptive/secretory activity as well as enteric motor activity in the gut (Christofi et al., 2001; Antonioli et al., 2008b).
Intestinal disorders, with particular regard for inflammatory bowel diseases (IBDs), are associated with significant alterations of gastrointestinal motor, secretory and sensory functions as well as abdominal discomfort/pain (Linden et al., 2003). Increasing evidence supports the concept that abdominal symptoms in patients with IBD depend on amplified interactions between the immune system and enteric neural circuitries and that adenosine participate in the modulation of these motor functions under inflammatory conditions (Kucharzik et al., 2006, Linden et al., 2003). Adenosine can modulate inflammatory responses in different ways and through different cellular types, which are implicated in phlogistic reactions and express adenosine receptors. In these cells, adenosine can block the release of cytokines as well as the production of superoxide anion (Thiel et al., 2003; Antonioli et al., 2008a), and studies, conducted on experimental models of inflammation or septic shock, have demonstrated a contribution of purinergic receptors in the modulation of gut inflammatory responses (Haskò and Cronstein, 2004).
Siegmund et al. (2000) demonstrated the ability of adenosine to ameliorate significantly experimental colitis in rats treated with GP515, an adenosine kinase inhibitor, thus corroborating the hypothesis of an
anti-43
inflammatory role played by this autacoid. Moreover, Odashima et al. (2005) observed the efficacy of ATL-146e, a selective A2A receptor agonist, in reducing inflammatory parameters in a model of experimental ileitis in rabbits. In the same line, Cavalcante et al. (2006) demonstrated that the new A2A receptor agonist ATL 313 reduced tissue injury and inflammation in mice with enteritis induced by Clostridium difficile toxin A.
In humans, there is evidence to suggest an involvement of adenosine pathways in the anti-inflammatory effects exerted by drugs employed in the clinical management of chronic inflammatory diseases, including IBDs (sulfasalazine, methotrexate). In particular, Cronstein et al. (1999) provided initial evidence supporting the ability of methotrexate and sulfasalazine to promote the release of adenosine from a variety of cells and tissues subjected to adverse conditions through an increment in the 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR).
44
INFLAMMATORY BOWEL DISEASES
Ulcerative colitis (UC) and Crohn‟s disease (CD), collectively known as inflammatory bowel disease (IBDs), are severe and debilitating disorders which produce a wide range of gastrointestinal and extradigestive symptoms, with great negative impact on patient‟s quality of life (Hanauer and Present, 2003). Although the precise etiology, leading to the onset of these chronic inflammatory conditions, remains unclear, the knowledge of molecular mediators and mechanisms implicated in tissue injury has been greatly expanded, and certain features of these diseases have suggested several areas of possible pathogenic importance, such as genetic, infectious, immunological and inflammatory factors (Ardizzone and Porro, 2002). Several lines of evidence suggest that CD and UC result from a genetic predisposition, with the involvement of multiple susceptibility genes, some of which are common to both diseases and some are linked separately to one disease or the other. For example, genetic factors play a pivotal role mainly in CD than in UC (Shanahan, 2001). In particular, an association between Crohn‟s disease and the presence of genomic regions containing nucleotide oligomerization domain 2 autophagy genes (NOD2) has been demonstrated. In 1996, NOD2 was identified by genome-wide screening on the chromosome 16 in families with several affected members, and mutations of this gene in this region have been conclusively
45
associated with Crohn‟s disease. NOD proteins are thought to be cytosolic receptors for pathogenic bacterial signals. NOD2 is expressed in monocytes and activates nuclear factor-kB (NF-kB), which is a key transcriptional factor involved in the onset of immuno-inflammatory responses. These mutations were found in 8% of patients with familial Crohn‟s disease, as compared with 4% of controls. Mutations were not more frequent in patients with UC than in controls, thus supporting the hypothesis that CD and UC are related, but distinct disorders (Ardizzone and Porro, 2002).
Among the several factors related with IBD pathogenesis, one of the least understood is represented by the environment. The importance of this factor is supported by the increasing frequency of CD cases in the more-developed world, and from the rising incidence of this disease observed in new developed countries. Environmental factors that might affect the development of mucosal immune system, the enteric microflora, or both, include: improved hygiene conditions, consumption of sterile and non-fermented foods, and vaccination (Shanahan, 2002).
Another environmental factor that seems to affect the disease phenotype is cigarette smoking. In this regard, cigarette smoking has been shown to affect cellular and humoral immunity (Miller et al., 1982; Srivastava et al., 1991), and to increase colonic mucus production (Cope et al., 1986).
46
Moreover, both smoking and nicotine reduce colonic motility (Coulie et al., 2001). Finally, results obtained from in vivo studies have shown an inhibitory effect of nicotine on Th-2 cells function, which predominates in UC, while no effect has observed in Th-1 cells, typical of CD (Madretsma et al., 1996).
The psychological stress, as observed in the 40% of patients with UC, represents another potential trigger (Theis and Boyko, 1994). Indeed, substantial evidence links psychological stress with increased illness and, possibly, increased susceptibility to infections through stress-related impairment of functional immune responses (Herbert and Cohen, 1993).
Defects in mucosal barrier may also be involved in the pathogenesis of IBD. In some individuals, the overt inflammatory response may be an appropriate response to an excessively permeable intestinal barrier. This barrier defect may be inherent or induced by intercurrent infections or pharmacological treatments (i.e., nonsteroidal anti-inflammatory drugs). The loss of tolerance to resident microbial flora is considered a central event in the pathogenesis of IBD. Indeed, a defective induction of oral tolerance has been demonstrated in IBD patients (Kraus et al., 2004). The available data do not indicate a single, persistent pathogen as the universal cause of IBD. However, during the last few years, Mycobacteria
47
implicated in the pathophysiology of IBD, but conclusive data are still lacking (Ardizzone and Porro, 2002). The resulting infiltration of gut wall by granulocytes and macrophages leads to the release of enzymes, reactive oxygen intermediates, and proinflammatory cytokines, which then cause an extensive damage to the intestinal tissue (Brown and Mayer, 2007).
Under physiological conditions, a continuous, low grade of “controlled” inflammation is a characteristic feature of the gastrointestinal tract. This is a reflection of the fact that the intestine has a large surface area, which is continuously exposed to dietary antigens and microorganisms. The intestinal lamina propria contains a complex population of immune cells that balance the requirement for immune tolerance of luminal microbiota with the need to defend against pathogens, the excessive entry of luminal microbiota, or both (Figure 12A). The hallmark of active inflammatory bowel disease is a pronounced infiltration of innate immune cells (neutrophils, macrophages, dendritic cells, and natural killer T cells) and adaptive immune cells (B cells and T cells) into the lamina propria. T cells normally constitute one-third of cells in the intestinal lamina propria, and the phenotypic distribution of CD4+ and CD8+ T cells is similar to that of peripheral blood lymphocytes, with a preponderance of CD4+ (MacDonald and Pender, 1998).
48
This complex interaction between genetic, microbial and environmental factors promotes the activation of different immune cellular populations at the level of intestinal mucosa, facilitated by alterations in the enteric epithelial barrier, with the appearance of an inflammatory process and subsequent tissue damage (Ardizzone Bianchi Porro, 2005) (Figure 12B).
Figure 12. The intestinal immune system in health (A) and disease (B) (Abraham et al.,
49
50
INTRODUCTION
Inflammatory bowel diseases (IBDs) are severe chronic disorders associated with periods of improvement followed by relapse that affect the gut (Torres and Rios, 2008). They are characterized by a wide range of gastrointestinal and extra-digestive symptoms, including diarrhea, rectal bleeding, abdominal pain, weight loss, skin and eye disorders (Irvine, 2004), as well as delayed growth and sexual maturation in children (Diefenbach e Breuer, 2006; Biank et al., 2007). The incidence of these disorders is highest in Western countries, where they particularly affect children and young adults. Indeed, IBDs are firstly diagnosed in people with age ranging from 15 to 25 years, and they reach another peak of incidence usually at the age of 50, thus representing a highly relevant social burden (Hanauer and Present, 2003; Ardizzone and Porro, 2005).
IBDs greatly alter the quality of life, and the progression and complication of these pathologies often require extensive and invalidating surgical procedures, with a negative impact on patient‟s well being and residual gastrointestinal functions. Indeed, both CD and UC are characterized by recurrent and severe inflammation of enteric mucosa at different levels of the gastrointestinal tract, associated with alterations of gastrointestinal motor, secretory and sensory functions, as a consequence
51
of structural and/or functional changes in the enteric nervous system
(Blandizzi et al., 2003; Lomax et al., 2005).
At present, pharmacotherapy represents the mainstay of IBD management, and most of currently available drugs aim to relieve symptoms, decrease the inflammatory response, promote lesion healing, and prevent relapse (Stenson and Snapper, 2008). Against the high and increasing rate of IBD occurrence, a large body of evidence highlights the scarce efficacy and/or safety of traditional drugs (including corticosteroids, immunosuppressants and salycilates). Likewise, other therapeutic options, based on treatments with biotechnological drugs, display some effectiveness, but they are also characterized by severe adverse effects. Overall, the pharmacological therapies available for treatment of IBDs are far from being satisfactory, and intensive efforts are currently in progress to search for new drugs endowed with favourable benefit/risk profiles. In this regard, increasing evidence suggests the adenosine system as an attractive target for development of novel drugs against gut inflammation (Burnstock, 2008; Antonioli et al., 2008a; Antonioli et al., 2008b).
Initial demonstrations of the immunomodulatory actions mediated by extracellular adenosine, date back to 1970s. Recent in vitro and in vivo studies clearly confirm the beneficial role of this autacoid as an immune modulator. Indeed, adenosine is released in the proximity of immune cells
52
in tissues subjected to various forms of injurious stimuli, including ischemia and inflammation. In the majority of experimental systems, the immunosuppressive effect of adenosine results from the occupancy of its receptors expressed on various immune cell populations (lymphocytes, neutrophils, monocytes, macrophages, and dendritic cells). In addition, it has been showed that the removal of endogenous adenosine signaling exacerbates immune activation, and consequently aggravates tissue dysfunction following acute injurious stimuli (Hasko and Cronstein, 2004; Antonioli et al., 2008a).
All the above observations have stimulated the research of novel drugs suitable for treatment of IBD through the pharmacological modulation of adenosine pathways (Siegmund et al., 2001; Odashima et al., 2005; Guzman et al., 2006; Cavalcante et al., 2006; Antonioli et al., 2007). However, beside the clinical benefits that adenosine may provide on bowel inflammation, the narrow therapeutic index and/or the lack of receptor selectivity of the endogenous nucleoside account for the occurrence of adverse effects on the cardiovascular (tachycardia and hypotension) as well as central nervous system, as previously mentioned. In this regard, efforts are focusing on the search of new drugs able to increase the endogenous concentration of adenosine at the site of inflammation, either indirectly or directly through the local stimulation of
53
its receptors in order to avoid the systemic side effects associated with the actions of adenosine on the periphery.
AIM OF THE RESEARCH PROGRAM
Based on these premises, the present research program has been implemented in order to:
1) Evaluate the anti-inflammatory efficacy of the novel adenosine deaminase inhibitor 4-amine-2-(2-hydroxy-1-decyl)pyrazole[3,4-d]pyrimidine (APP) in the setting of 2,4-dinitrobenzenesulfonic acid (DNBS)-induced colitis, analyzing adenosine deaminase expression in inflamed colonic tissues and characterizing the receptors subtypes involved in the ameliorative effects of endogenous adenosine
2) Examine the effects of pharmacological activation of AMPK by acadesine in a rat model of experimental colitis induced by DNBS and evaluate the underlying anti-inflammatory mechanisms
3) Examine the effects of PSB0777, a novel and scarcely absorbable A2A receptor agonist, in a rat model of experimental colitis induced by oxazolone
54
MATERIALS AND METHODS Animals
Albino male Sprague-Dawley rats (250-300 g body weight) (Harlan Italy, UD) were employed throughout the research program.
Animals were fed standard laboratory chow and tap water ad libitum and were not subjected to experimental procedures for at least 1 week after their delivery to the laboratory. They were housed (five in each cage) in temperature-controlled rooms on a 12-hour light-dark cycle at 22-24°C and 50-60% humidity. Their care and handling were in accordance with the provisions of the European Union Council Directive 86-609 recognized and adopted by the Italian Government.
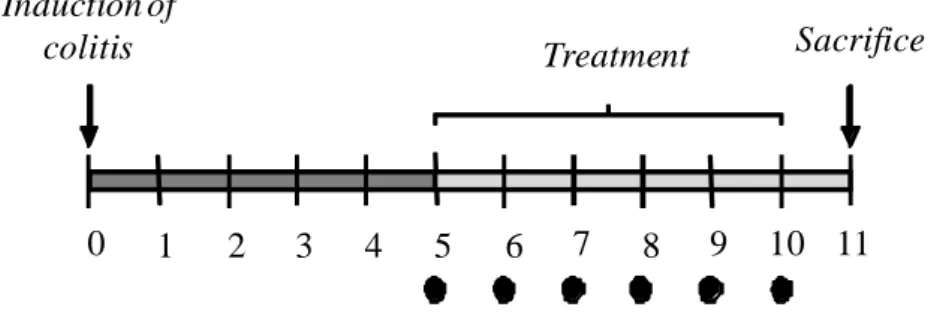
PART 1. STUDY OF THE ANTI-INFLAMMATORY ACTIVITY OF THE NOVEL ADENOSINE DEAMINASE INHIBITOR APP IN AN EXPERIMENTAL MODEL OF COLITIS
Structural characteristics of APP
The novel adenosine deaminase inhibitor APP, synthesized in the laboratory of Prof. Federico Da Settimo (Department of Pharmaceutical Sciences, Faculty of Pharmacy, University of Pisa), is an erythro-9-(2-hydroxy-3- nonyl)adenine (EHNA) analogue in which the adenine base was substituted by the 4-aminopyrazole [3,4-d]-pyrimidine system. The
55
synthesis of APP was performed as previously reported (Da Settimo et al., 2005). In brief, commercially available 3-amino-4-pyrazolecarbonitrile was alkylated with 1,2-epoxydecane in dimethylformamide at 100°C in the presence of K2CO3. The resulting compound was then cyclized with boiling formamide to yield the compound APP.
In vitro adenosine deaminase inhibition assay
In vitro activity of adenosine deaminase was determined spectrophotometrically by monitoring, for 2 min at 262 nm, the decrease in the absorbance resulting from deamination of adenosine into inosine by the enzyme adenosine deaminase (Da Settimo et al., 2005). The enzyme activity was assayed at 30°C in a reaction mixture containing 50 µM adenosine, 50 mM potassium phosphate buffer (pH 7.2), and 0.3 nM adenosine deaminase in a total volume of 500 µl. The inhibitory activity of EHNA and APP was assayed by adding 100 µl of drug solution to the
N N NH2 N N OH N N NH2 N N OH APP EHNA