PhD Course in Agrobiosciences
Academic Year
2016-2017Evaluation of microalgae and
Ascophyllum nodosum extracts as
biostimulants for abiotic stress
mitigation.
Author
Francesco Gresta
Supervisor
2
This research has been done on the projects 85/2014 on 3/3/2014 and 443/2014 on 24/11/2014 funded by the company Valagro S.p.A.; the agreement with Valagro regulates the intellectual property of the results. The current document is confidential. Anyone reads the document or attends to the oral presentation of the results is held to respect the confidentiality of the data and any other information.
Part of this study has been extracted from the paper, recently accepted in Frontiers in Plant Science, specialty edition Plant Abiotic Stress, titled “Ascophyllum nodosum seaweed extract alleviates drought stress in Arabidopsis by affecting photosynthetic performance and related gene expression”. A.Santaniello, A.Scartazza, F. Gresta, E. Loreti, A. Biasone, D. Di Tommaso, A. Piaggesi, P.Perata.
3
Acknowledgements
Firstly, I would like to thank Prof. Pierdomenico Perata for giving me the opportunity to work in Plant Lab and for the support in overcoming numerous obstacles I have been facing through this research.
My real thanks also go to Dr. Elena Loreti, Dr. Antonietta Santaniello and Dr. Andrea Scartazza for supporting my work and help me to get results. Without the precious support, this work would never have been possible.
I am grateful to Joshua Browne for the significant help in writing, for his feedback and positivity that he passed to me during the drafting of the thesis.
I would like to thank the two revisors of the thesis Prof. Francesco Licausi and Prof. Alberto Pardossi who gave much valuable advice in the last step of this
document.
I thank my fellow lab mates for the nice time we spend in the lab, for the support in weekend and nights we were working together, and of course friendship.
I am particularly grateful to Plant Lab staff, for their help, valuable suggestions and discussions.
Also, I thank the friends and staff of plant physiology group-department of agriculture, food and environment of the University of Pisa, for their technical and moral assistance during the experimental work.
Last but not the least, I would like to thank my family: my parents and my brother for supporting me throughout my Ph.D. and my life in general.
4
Abstract ... 6
1 Introduction ... 7
1.1 Plant Biostimulants ... 8
1.1.1 Microalgae ... 9
1.1.2 Ascophyllum nodosum (L.) Le Jol. ... 10
2 Literature Review ... 12
2.1 Plant responses to drought ... 12
2.2 Drought stress genes and their regulation ... 13
2.3 Physiological response to drought ... 18
2.3.1 Gas exchange inside plants ... 18
2.3.2 Water Use Efficiency ... 20
2.3.3 Stomatal Conductance ... 20
2.3.4 Mesophyll conductance ... 21
2.4 Photosynthesis damage during drought ... 22
2.5 Salt stress ... 25
2.5.1 Salt stress signaling ... 26
3 Objective of the thesis ... 28
4 Microalgae extracts ... 29
4.1 Material and Methods ... 29
4.1.1 Plant material and growth conditions ... 29
4.1.2 Drought stress ... 32 4.1.3 Salt Stress ... 33 4.2 Results ... 35 4.2.1 Drought stress ... 35 4.2.2 Salt stress ... 38 4.3 Discussion ... 42
5
5 Ascophyllum nodosum extracts ... 43
5.1 Material and Methods ... 43
5.1.1 Plant material and growth conditions. ... 43
5.1.2 Drought stress ... 47
5.2 Results ... 52
5.2.1 Ascophyllum SEA-line extracts screening ... 52
5.2.2 Ascophyllum nodosum SEA11 molecular and physiological responses to drought stress ... 62
5.3 Discussion ... 73
5.3.1 Ascophyllum nodosum treatments and timing screening ... 73
5.3.2 Ascophyllum SEA11 molecular and physiological response ... 75
6 Conclusion ... 78
6
Abstract
Drought and salinity are two of the major factors affecting crop productivity. A challenge in the field of food security is developing strategies to grow crops productively in increasingly extreme environments. The use of organic inputs to enhance abiotic tolerance in plants has been subject to increasing investigation, and seaweed extracts, from micro- and macro-algae, are the most promising, introduced so far in agriculture. They belong to a specific product category named biostimulants, which promote plant growth and development throughout the full crop cycle. These beneficial effects include increasing crop yield, general crop and fruit quality, improving tolerance, recovery from abiotic stress and enhancing nutrient uptake. The research for new potential biostimulants components, among biological organisms, and the investigation about their mechanisms of action represent an interesting topic of research. The object of this work was to evaluate the effects of some microalgae extracts, and one of the most used macro-algae extracts,
Ascophyllum nodosum, for improving drought and salt stress tolerance in plants. Thirty-six
prototypes, obtained from nine microalgae strains and four prototypes, obtained from
Ascophyllum nodosum, were tested. This study has been conducted in collaboration with
Valagro S.p.A. (Atessa, CH, Italy), who developed and provide the prototypes. The tolerance tests to drought and salt stress were performed using Arabidopsis thaliana plants, grown in hydroponic system. Interestingly the results showed that, among the investigated microalgae strains, one prototype from H.pluvialis and two prototypes from
Spirulina sp. induced respectively drought and salt stress mitigation in Arabidopsis plants.
Four Ascophyllum nodosum prototypes (SEA7-11-13-14) were screened for preliminary drought stress tolerance induction on treated plants. According to the results, obtained from these preliminary tests, the research was focused on just one Ascophyllum nodosum prototype, named SEA11. A secondary screening was developed, in order to evaluate a possible field application of Ascophyllum nodosum SEA11 prototype, and two different methods of application were evaluated, root drench and foliar spray. The results showed a significant mitigation increased in treated plants, compared to the untreated control. Finally, the mode of action of Ascophyllum nodosum SEA11 was investigated. In order to study the physiological responses in plants treated with this product, leaf gas exchange parameters were measured. Moreover, the expression of genes correlated with stomatal movements and mesophyll conductance, such as genes involved in the protection of photosynthesis machinery, in ABA-responsive and ROS signaling were analysed. We found that treatment with Ascophyllum nodosum SEA11 induced a partial stomatal closure, associated with changes in the expression levels of genes involved in ABA-responsive and antioxidant system pathways. The pre-activation of these pathways results in a greater ability of Ascophyllum nodosum SEA11-treated plants to maintain better photosynthetic performance compared to control plants throughout the dehydration period, combined with a higher capacity to dissipate the excess of energy as heat in their photosystem II reaction centers.
7
1 I
NTRODUCTION
Food resources increased substantially over the past few decades, thanks to the development of agricultural technologies of the last century (Alexandratos and Bruinsma, 2003; Foley et al., 2011). Between 1965 and 2005, the percentage of people that had sufficient food supply has almost doubled, demostrating that the use of agricultural lands has become steadily more efficient (Porkka et al., 2013). However, the scenario in the next future is not so promising, considering how climate changes influence agricultural efficiency. Because of the global warming, the agricultural production of certain regions such as South-Central Asia, Central Europe and South-Est of United States is increasingly exposed to drought stress, due to daily temperature extremes and heat waves (Carrao et al., 2016; IPCC, 2014). Another definite challenge of the future is the constant growing population, which is expected to reach 9 billion people by 2050, placing even greater demand on the limited resources. One is the blue water, which is present in rivers and aquifers (Eliasson, 2015) and in many regions, with expanding agriculture, its use is over the sustainable limits (Steffen et al., 2015).
Another important abiotic stress, limiting growth and yield of crop plants, is salt stress. The massive application of soluble fertilisers, soil amendments and poor quality irrigation water have contributed to the salinization of soil. Even seawater intrusion (a growing problem as sea levels rise in many parts of the world) deposits a large amount of salt in coastal soils (Rengasamy, 2010). Since almost 20% of irrigate land is salt affected, salt stress is recognised as being an important abiotic factor for modern agriculture (Pitman and Läuchli, 2002; Wicke et al., 2011).
Considering the general trends of an increasing demand for water and rising salinity, solutions to these problems must be developed.
Plants respond to drought and salt with a series of physiological processes: closing the stomata to regulate the plant’s water use efficiency (WUE), inhibiting cell growth, photosynthesis and reducing plant biomass. Changes at the transcriptome level occur during stress mainly affecting the expression of genes involved in stress response pathways (Shinozaki and Yamaguchi-Shinozaki, 2007).
Various approaches have been suggested to use molecular breeding and genetic engineering to induce improved abiotic stress tolerance (Vinocur and Altman, 2005). However, these methods suffer from a number of pitfalls. Molecular breeding is a laborious and time-consuming process, while genetic engineering is limited by a lack of global acceptance, restrictive and costly GMO regulation (Kanchiswamy et al., 2015).
Use of organic inputs to enhance abiotic tolerance in plants has been subject of study, and seaweed extracts are among the most promising organic inputs that have been discovered so far (Khan et al., 2009; Sharma et al., 2014). Seaweed extracts have been used for centuries as fertiliser, stress-resilience enhancer and plant growth regulator (Crouch and Van Staden, 1992; Fan et al., 2011; Rayirath et al., 2009; Wally et al., 2013). They are in a class of agronomic products known as plant biostimulants (du Jardin, 2015) and their use
8
in agricultural production is a common practice. In particular, Ascopyllum nodosum is the most used, as well as the most thoroughly studied (Khan et al., 2009).
The study of new potential compounds for new biostimulants formulation constitutes an interesting topic of research.
1.1 P
LANTB
IOSTIMULANTSPlant biostimulants are substances between a plant-protection product and a fertiliser. However, they are not a plant protection product, because they act only in improving plant vigor, without offering protection against pests, nor are they a fertiliser, because they do not produce nutritional activity (La Torre et al., 2016).
The industrial consortium of biostimulants producers in Europe (European Biostimulants Industry Council; EBIC) introduced for the first time the definition of plant biostimulants. They are considered plant growth and development promoter throughout the full crop cycle. These beneficial effects include increasing crop and fruit quality, improving tolerance to and recovery from abiotic stress and enhancing nutrient uptake. Also the physiochemical properties of soil generally are improved, since they stimulate beneficial microbial activity. Finally, the EBIC specifies that biostimulants can contain nutrient elements, but that they are not a fertiliser, because biostimulants operate with a different mechanism (European Biostimulants Industry Consortium, 2011).
Several scientific studies demonstrate the benefits of plant biostimulants. These include improved yields, increased chlorophyll contents, resistance to abiotic stresses, reduced use of N- fertiliser (Kunicki et al., 2012; Paradiković et al., 2011; Petrozza et al., 2014; Richardson et al., 2004; Spinelli et al., 2010; Vernieri et al., 2006).
Currently, biostimulants in the European Union (EU) do not have a clear legal status as they are regulated by national laws, substantially different from one another.
La Torre et al. (2016) reviewed all the European Union member’s regulations of biostimulant products and the possible future scenario. In some member states biostimulants legislation is included in the Regulation EC No 1107/2009, that concerns the distribution of plant protection products in the market, or among some member states by Regulation EC No 2003/2003. These generate a highly variable regulation for the introduction of biostimulant products to the European market. In some countries, the regulatory process is free-access to the market (United Kingdom), or requires a pre-market notification to the relevant authority (Denmark, Spain, Netherlands). Other member states require scientific data to be presented during notification procedures (Germany, Belgium, Italy, Austria) and with only a rigorous authorisation process (France, Hungary, Slovakia, Czech Republic). To align the regulatory process of biostimulants inside the EU, the Working Group on Fertilisers of the Europen Commission is developing a draft definition of plant biostimulants, but a final definition is yet to be established.
9
1.1.1 Microalgae
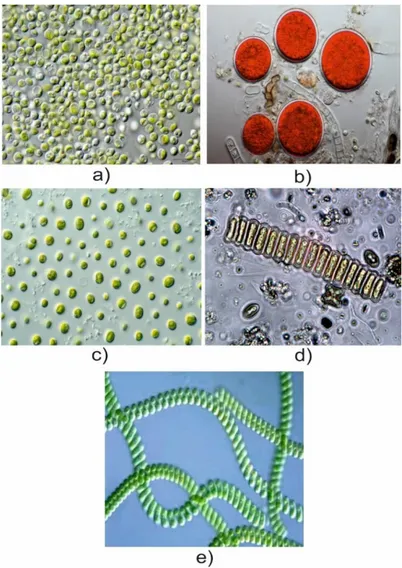
Microalgae are microscopic algae typically found in fresh and salt waters (Thurman and Burton, 1997). In contrast to aquatic plants, microalgae do not have embryos, roots, stems, or leaves (Figure 1). They do however have the competencies to use water, sunlight and CO2 to synthesise biomass through photosynthesis (Richmond, 2008). These
competencies make microalgae interesting for high-market value biomass production, requiring only inexpensive resources like water, fertilisers, and sunlight. Many companies operate at an industrial scale to produce microalgae biomass for applications in cosmetics, animal-feed and pharmaceuticals (Zhu, 2015).
In industrial plants, phototrophs including microalgae are grown in photobioreactors (PBR) (Tredici, 2002). Two distinct systems have been developed and are based on the open pond and closed photobioreactor technologies (Acién Fernández et al., 2013).
Most studies in microalgae, tended to focus on biodiesel production from microalgae biomass (Chisti, 2007). Nannochloropsis sp. and Chlorella sp. have been receiving much attention as a potential biodiesel source due to the high amount of lipids that can accumulate (Converti et al., 2009; San Pedro et al., 2014). However, also biochemically active molecules from microalgae raise considerable interest (Milledge, 2011). The carotenoid astaxanthin produced by Haematoccosus pluvialis is the most relevant, considering its several benefits to human health (Guerin et al., 2003; Lorenz and Cysewski, 2000). Scenedesmus sp. is also known for the high production of lutein, a high market-value molecule (Fernández-Sevilla et al., 2010; Sánchez et al., 2008).
The cyanobacteria (blue-green algae) have been identified as one of the most promising groups of organisms from which active natural product can be isolated (Singh et al., 2005). Many companies are producing food supplements from Spirulina sp. biomass, considering its high protein content and nutrient value (Khan et al., 2005; Spolaore et al., 2006).
10
Figure 1.) Microalgae strains: a) Chlorella vulgaris (Eigenes Werk, cc by-sa 3.0); b) Haematococcus pluvialis (Frank Fox, cc by-sa.de); c) Nannochloropsis sp. (csiro, cc by 3.0); d) Scenedesmus bijunga (pd-epa); e) Spirulina platensis (esa, cc by-sa 3.0 igo).
1.1.2 Ascophyllum nodosum (L.) Le Jol.
Ascophyllum nodosum is a brown seaweed of the Fucaceae family and is the only
member of the genus Ascophyllum (Figure 2). The group of Brown algae (Phaepyceae) contains 265 genera and 1500-2000 species (Bold and Wynne, 1985). Their unique color is produced by the large amount of the carotenoid fucoxanthin (Lee, 2008). According to the classification of Bold and Wayne there are 13 orders in the Phaepyceae family (Bold and Wynne, 1985). However, only two are relevant: the Laminares and Fucales (Davis et al., 2003), which include Ascophyllum nodosum.
Ascophyllum nodosum is present in the coastal waters along the North Atlantic coasts and
on the northwest coast of Europe (Ugarte et al., 2007). Their growth is bifurcated, generating long fronds with egg-shaped air-bladders in series at regular intervals (Hiscock, 1979; Thomas, 2002; Ugarte and Sharp, 2001).
11
Figure 2.) Ascophyllum nodosum (L.) Le Jol. (PD-Francesco Gresta).
This brown alga is well known for its extracts used in biostimulants manufacturing. Usually, the seaweed is harvested with a machine, then it is washed with fresh water, chopped and oven dried (Boney, 1965; Roberts and Upham, 2012). Then an aqueous process is used for extracting a water-soluble fraction with low pressure, or with strong acid or alkali treatment (Sharma et al, 2014).
The Ascophyllum extracts enhance seedling emergence, plant vigor, chlorophyll content and resistance to biotic stress (Khan et al., 2009) and protect against freezing and salt stress (Jithesh et al., 2012; Nair et al., 2012). Moreover, it can mitigate the effects of drought on the orange tree, creeping bentgrass and spinach (Spann and Little, 2011; Xu and Leskovar, 2015; Zhang and Ervin, 2004). However, the precise mechanism by which the seaweed extract can improve plant physiology is not yet completely understood, and the majority of the components of seaweed extract are yet to be defined (Wally et al., 2013).
12
2 L
ITERATURE
R
EVIEW
2.1 P
LANT RESPONSES TO DROUGHTPlants can perceive abiotic stresses and elicit appropriate responses with altered metabolism, growth and development. Many genes respond to drought stress with changes at the transcriptional level, driving the processes whereby the plant responds to the initial stress-factors (Chaves et al., 2003). The final responses of a plant to drought usually involve different mechanisms such as “escape”, “avoidance”, and “tolerance” (Bacelar et al., 2012), or the combination of all these mechanisms (Chaves et al., 2003). Drought escape refers to the most severe plant response to drought, investing all of its energies in reproduction. The plant shortens its life cycle, increases rate of growth and gas exchange, making full use of whatever resources are available (Maroco et al., 2000; Mooney et al., 1987). More recent evidencees (Riboni et al., 2016) show that drought escape requires the activation of photoperiodic gene GIGANTEA and the florigen genes FLOWERING LOCUS T and TWIN SISTER OF FT (TSF).
Drought avoidance occurs minimising water loss, closing the stomata, reducing light absorbance by rolling the leaf and decreasing leaf area. It can maximises water uptake by promoting root growth, moving nutrients stored in old leaves and increasing the rate of photosynthesis (Chaves et al., 2003). One of the most significant physiological plant responses for avoiding water loss is to optimise water use efficiency (WUE).
Plant drought tolerance is the result of a coordination of physiological and biochemical modifications at the cellular and molecular level. Most drought tolerance mechanisms involve protective proteins such as dehydrins and late-embryogenesis-abundant proteins (LEA proteins). It is well known that these proteins are accumulated under drought stress or other abiotic stresses and in seed development (Close, 1997). However, many functions of dehydrin and LEA proteins are unknown. Bravo et al. (2003) and Hara et al. (2001) proposed LEA proteins and dehydrins act like chaperons that protect proteins and membrane structure. Under drought stress, compatible solutes such as proline and betaine can protect proteins and membranes (Hincha and Hagemann, 2004). Damage of reactive oxygen species (ROS) during drought tolerance is limited by several detoxification mechanisms. Superoxide radicals (O2-), single oxygen (O21), hydrogen peroxidase (H2O2),
an alkoxy radical (RO) and hydroxyl radicals (OH-) are the main ROS produced in abiotic
stresses (Apel and Hirt, 2004). ROS react with lipids, protein, and other molecules, generating oxidative damage (McCord, 2000). Antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), glutadione reductase (GR) and ascorbate peroxidase (APX) increase in plants under drought stress in order to limit oxidative damages (Farooq et al., 2009; Osakabe et al, 2014). Also the flavonoid pigment antochyanins has been recognised as important components of the drought tolerance mechanisms. It is well known that antochyanins has a role in photosynthesis machinery
13
protection, in preventing osmotic stress, for its remarkable antioxidative activity (Chalker-Scott, 1999).
2.2 D
ROUGHT STRESS GENES AND THEIR REGULATIONDrought induces several genes in different plant species. The drought-inducible genes are mainly grouped on the basis of their function. One group is represented by functional genes such as, chaperones, LEA proteins, detoxification enzymes, water channels and enzymes involved in compatible solutes biosynthesis such as proline (Shinozaki and Yamaguchi-Shinozaki, 2007; Szabados and Savouré, 2010). There are many LEA and dehydrin-like genes in Arabidopsis thaliana, including responsive to desiccation (RD),
cold-regulated (COR), cold-inducible (KIN), low-temperature induced (LTI) and responsive to ABA (RAB) (Lång and Palva, 1992; Zhu, 2002). Major detoxification genes encode for
ROS antioxidant enzymes, such as superoxide dismutase (SODs), catalase (CAT) and ascorbate peroxidase (APXs). They are upregulated during drought (Das and Roychoudhury, 2014), as the genes that encode for the two main biosynthetic enzymes for anthocyanins, dihydroflavonol reductase (DFR) and leucoanthocyanidin dioxygenase/anthocyanidin synthase (LDOX/ANS) (Berdeja et al., 2015; Castellarin et al.,
2007; Nakabayashi et al., 2014).
The second group is represented by regulatory genes that code various components of drought signalling and transcription factors (Shinozaki and Yamaguchi-Shinozaki, 2007). Evidences indicate that abiotic-responsive genes are under complex regulation. Signalling pathways consist of regulatory networks of transcription factors (TFs), which bind short DNA sequences (cis-elements) to the regulatory parts (promoters) of the target gene, regulating expression, due to hormonal or environment stimuli (Nakabayashi et al., 2005). Drought response is triggered by the phytohormone abscisic acid (ABA). The biosynthesis of ABA is quickly induced by drought, activating the expression of 9-cis-epoxyncarotenoind
dehydrogenase 3 (NCED3), that encode for the main enzyme in ABA biosynthesis in the
vascular parenchyma cells of Arabidopsis thaliana (Endo et al., 2008; Iuchi et al., 2001). ABA activates a network of signalling pathways that starts with ABA perception and continues with a downstream signalling cascade, resulting in the final physiological responses.
The ABA signalling model is composed by pyrabactin resistance/pyrabactin resistance1-like/regulatory component of ABA receptors (PYR/PYL/RCARs) and a protein phosphatase-kinase complex type 2C protein phosphatase (PP2C)-SNF1-related protein kinase 2 (SnRK2) (Figure 3). The ABA is perceived by the receptors PYR/PYL/RCARs and the complex ABA-PYR/PYL/RCARs interacts and inhibits the dephosphatase activity of the protein phosphatase PP2C. The SnRK2 is then released from negative regulation by PP2C and promotes the phosphorylation of downstream transcription factor ABA-responsive element (AREB), binding protein/ABRE binding factors (AREB/ABF) and the phosphorylation of membrane ion channels (Umezawa et al., 2010). The phosphorylated transcription factors bind to cis-active elements of the promoter in the drought-inducible gene and promote the gene expression.
14
.
Figure 3.) Model of ABA signalling pathway. a) in normal condition PP2C inhibits SnRK2 by interaction and dephosphorization of SnRK2. Under drought stress the ABA binds to PYR/PYL/RACR and this complex interact with PP2C to inhibit the dephosphorisation activity of PP2C. SnRK2 is released from PP2C and phosphorylate a transcription factor such as AREB/ABF or membrane proteins; b) in ABA insensitive mutant abi 1-1 miss the PYR/PYL/RACR binding protein and the SnRK2 remains inactive also in presence of ABA (Umezawa et al, 2010).
AREB/ABF are not the only transcription factors involved in ABA-drought stress response. Several TFs are also associated with the ABA-mediated gene expression during drought and includes the classes bZIP, AP2/ERF, MYB, NAC, HD-ZF, HD-Zip, bHLH, C2H2-ZF, B3, WRKY and NF-Y (Fujita et al., 2011).
Scientists have conventionally considered ABA as the major signal that can activate abiotic stress responses. However, many studies have shown that there are genes induced independently from ABA (Shinozaki and Yamaguchi-Shinozaki, 1997). Potential
cis-acting elements have been analysed comparing their expression profile with those of
the promoter sequence of stress-inducible genes (Shinozaki et al., 2003). The ABA-responsive element (ABRE) is a cis-acting element involved in the ABA-dependent gene expression in response to abiotic stress. Two ABRE motifs were identified in the RD29B gene controlling the ABA-responsive expression in Arabidopsis thaliana (Uno et al., 2000). The transcription factors, AREB/ABF can bind to ABRE cis-element activating the gene
15
transcription (Choi et al., 2000; Uno et al., 2000). RAB18 gene has been shown to be ABA-dependent due to the presence of two ABRE cis-elements (Lång and Palva, 1992).
RD22 has been shown to be another ABA-drought-responsive gene. Two transcription
factors, MYC and MYB have been found to bind to the cis-active element of RD22 and promote the transcription of the gene (Shinozaki et al., 2003). On the other hand, many studies have shown that there are genes induced independently from ABA (Shinozaki and Yamaguchi-Shinozaki, 1997). The dehydration-responsive elements (DRE)/C-Repeat (CRT) are cis-acting elements functioning in the ABA-independent pathway. Two AP2/ERF transcription factors, C-REPEAT-BIDING FACTOR (CBF)/DREB1 and DREB2 have been found to bind to DRE/CRT elements (Yamaguchi-Shinozaki and Shinozaki, 2005). In normal growth condition DREB proteins are inhibited by the transcriptional repressor GROWTH-REGULATING FACTOR 7 (GRF7). GRF proteins are putative transcription factors and GRF7 bind in to the DREB2A promoter inhibit the transcription of the gene (Kim et al., 2012). Indeed, under normal growth conditions, an ubiquitin-proteasome pathway is involved in the degradation of DREB2A protein. A ubiquitin E3 ligase DREB2A-INTERACTING PROTEIN (DRIP1) and its homologous DRIP2 target the DREB2A protein, promoting the 26S proteasome activity (Qin et al., 2008).
A number of studies supposed a possible cross-talk in ABA-dependent and independent signalling pathways. The analysis of DREB2A promoter revealed that the transcription of the protein is also regulated by AREB/ABF2, AREB/ABF4 and ABF3 during ABA signalling under drought stress (Kim et al., 2011). Lee et al. (2010), found that several AP2/ERF transcription factors such as DREB2A, interact with AREB1/ABF2, AREB2/ABF4 and ABF3. However, is still unknown what influence this interaction has on gene expression. Interestingly, Kim et al. (2012) showed that GRF7 has a role in repressing both the ABA-dependent and ABA-inABA-dependent transcription (Figure 4).
16
Figure 4.) Crosstalk between dipendent and indipendent pathway in drought stress. The key transcription factor in ABA-indipendent pathway DREB2A is induced by drought stress. DREB2A protein are regulated in the cell by ubiquitin E3 ligase DRIP1 and DRIP2 and by GRF7 repressor, under unstressed conditions. The crosstalk between ABA-dependent and independent pathway is due by the GRF7 repression in both pathways and in the evidence that AREB/ABFs promotes DREB2A transcription (Yoshida et al., 2014).
Also reactive oxygen species (ROS) have an important role in modulating the gene expression under abiotic stress. ROS can be sensed by different mechanisms such as unidentified ROS receptors, redox-response transcription factors and direct inhibition of phosphatases by ROS (Figure 5; Mittler et al., 2004).
A growing body of literature has studied the downstream components of ROS signalling, revealing that starts with Ca2+ spiking that activates a serine/threonine protein kinase
(OXI1). The activation of OXI1 induce a mitogen-activated-protein kinase 3 and 6 (MAPK3/6) cascade, that finally modulates the expression of different transcription factors involved in ROS stress response such as WRKY, zinc finger C2H2 (ZAT) and MYB families (Desikan et al., 2001; Rentel et al., 2004). It has been shown that the OXI1 is also activated by phosphoinositide-dependent kinase (PDK) throughout the phospholipase-C/D-phosphatidic-acid pathway (Anthony et al., 2004).
17
The activity of redox-response TF might influence the expression of OXI1 and MAPKs such as the expression of ROS stress response TF (Mittler et al., 2004). ROS accumulated in the cytosol can be sensed by different redox-response transcription factors such as the heat shock transcription factors Hsfs. The Hsfs are transcription factors that are rapidly induced by heat shock and bind to the heat shock element (HSE) in the promoter of the gene (Miller and Mittler, 2006). Hsfs are upregulated not only in heat stress but also during other abiotic stress such as drought. Several Hsfs have been shown to take part in the ROS gene network during abiotic stress. ROS that are accumulated in the cytosol could be perceived by different redox-response transcription factors such as HsfA4, which activate a cascade of other transcription factors such as ZAT family (ZAT12, ZAT10 and 7) and WRKY family (Miller et al., 2008). The zinc finger ZAT10 and ZAT12 transcription factors regulate the transcription of antioxidant enzymes such as ascorbate peroxidase1 (Apx1) and ascorbate peroxidase 2 (Apx2) in abiotic stress. Their up-regulation induces the expression of ascorbate peroxidases genes, increasing abiotic tolerance (Davletova et al., 2005; Mittler et al., 2006; Rizhsky et al., 2004).
Figure 5.) ROS signalling pathway scheme: ROS can be perceived by ROS receptors, redox-sensitive TF and by inhibition of phosphatases. The ROS perception by unknown receptors induced a Ca2+ spiking that activates the protein kinase OXI1 and the mitogen-activated protein kinase (MAPK) 3/6. The MAPK 3/6 induced the transcription of different transcription factors, involved in ROS responses. The inhibition of phosphatases might influence the activity of OXI1 and MAPK3/6. Redox-sensitive TF can also modulate the transcription of OXI1, MAPKs, and ROS responsive TF. In the figure is also indicated a possible ROS signaling pathway amplification mediated by NADPH, nitric oxide (NO) and salicylic acid (SA) (Mittler et al., 2004).
18
2.3 P
HYSIOLOGICAL RESPONSE TO DROUGHTPlants under drought stress react with several physiological responses at the molecular, cellular and whole-plant level (Bartels et al., 1996; Bray, 1993; Chaves et al., 2003). One of the most relevant physiological response, induced in drought avoidance, is the optimization of the water use efficiency (WUE). This response is of particular significance to this study, and so it has been examined in detail, in terms of molecular and physiological mechanisms.
2.3.1 Gas exchange inside plants
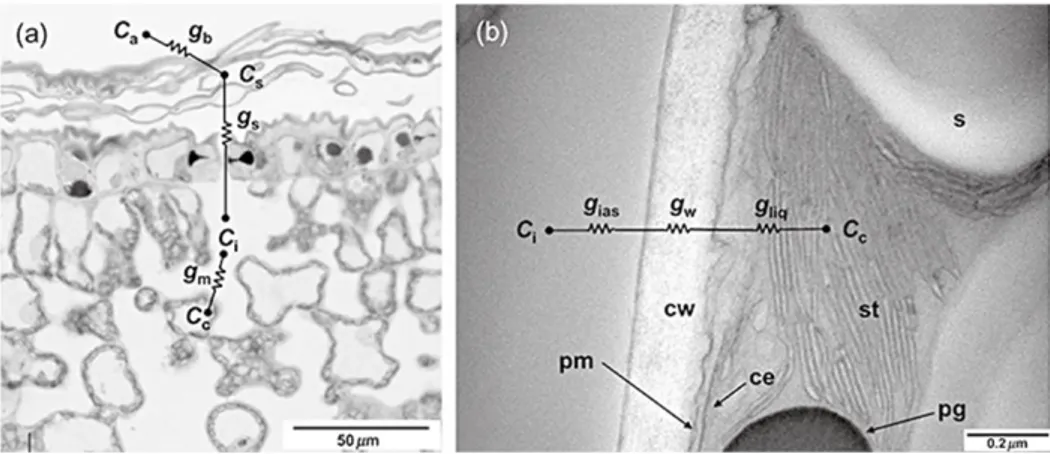
Plants need carbon dioxide (CO2) in order to photosynthesise, there are several obstacles in the leaf for CO2 diffusionin order to reach the site of carboxylation in the chloroplast (Figure 6).
Figure 6.) Micrograph of abaxial olive leaf: a.) CO2 diffuses from the atmosphere (Ca) to leaf surface (Cs) and intercellular air space (Ci) to the chloroplast (Cc). Boundary layer (gb), stomata (gs) and mesophyll (gm) conductance are indicated; b.) Electron micrograph of mesophyll cell in grapevine leaf, the CO2 diffusion from intercellular air space (Ci) to chloroplast (Cc) is composed by intercellular (gias), cell wall (gw) and liquid resistance (gliq) conductance. In this photo, it is possible to see the subcellular components of the mesophyll cell: cell wall (cw), plasma membrane (pm), chloroplast envelop (ce) and stroma thylakoid (st). A grain of starch (s) and plastoglobule (pg) can also be seen (Flexas et al., 2007).
The first diffusional obstacle or resistance (r) for CO2 transport is across a laminar
boundary layer to reach the leaf surface (rb). The second is stomatal resistance (rs): CO2
can only diffuse in the interior part of the leaf through the stomata cavity. Third, CO2 must
diffuse through the interior of the leaf through an interconnected intercellular air space (Ci).
Fourth, the CO2 has to enter the mesophyll cells (rm). Here CO2 diffusion occurs in both
gaseous and liquid phases. Several components of mesophyll resistance can be distinguished. The first element is the resistance to CO2 before entering into the mesophyll
19
cells (rias). When entering the cells, CO2 has to cross the cell wall (cell wall resistance; rw)
and dissolve in the liquid phase inside mesophyll cells (liquid resistance, rliq), to reach the
stroma of the chloroplast (Warren, 2008). Once in the stroma, the molecular CO2 is taken
up by the carboxylation enzyme ribulose-1,5-biphospate carboxylase oxygenase (Rubisco), starting the Calvin cycle. Usually, all the components of CO2 resistance
diffusion (rb, rs, rm) are expressed as conductance. The conductance is proportionally
reciprocal to resistance (g = 1/r) and is explained by Fick’s first law. In accordance with Fick, the direction of the diffusive flux of any particle is from high to low concentration. The Fick’s equation describes the flux between locations x1 and x2 with concentration C1 and
C2 at steady state:
φ = D
(x2 - x1)(C2 - C1 )
Where ϕ is the flux, D is the diffusion coefficient (m2s-1), a proportional constant between
the flux of a particle and its gradient. The ratio D/(x2 – x1) is also named conductance (g).
The diffusion of CO2 in a leaf follows Fick’s law consistently (Flexas et al., 2013):
AN = gs (Ca - Ci)
Where AN is the flux of CO2 in the leaf, or also indicated photosynthesis rate (mmol CO2 m -2s-1), gs is the stomatal conductance (mmol H2O m-2s-1), Ca is the CO2 concentration in the
ambient air and Ci is the intercellular CO2 concentration. The flux of CO2 can also be the
product of mesophyll conductance (gm) and CO2 concentration differential between
sub-stomatal cavities (Ci) and chloroplast (Cc):
20
2.3.2 Water Use Efficiency
Plant responses to drought can be improved by avoidance to drought stress, through the conservation of water by improving the water use efficiency (Amudha and Balasubramani, 2011). Water use efficiency (WUE), does not have a single definition. In most literature, WUE is the efficiency of carbon gained for water loss or it is expressed as efficiency over time such as biomass accumulation or yield harvested (Bacon, 2009).
In plant physiology WUE is defined as the mole of carbon gained through photosynthesis (AN) in exchange for water used in transpiration (E). The ratio AN/E is also referred to as
“instantaneous” water use efficiency (Condon et al., 2004). WUE can also be expressed as the ratio between AN and the stomatal conductance (gs), that is the “intrinsic” WUE (Parry
et al., 2005).
Under drought stress plants close the stomata in order to limit water loss with consequent reduction of gs, improving WUE. However, an increase in WUE due to gs results in less
carbon available for carbon fixation (AN), reducing crop-yield (Rockström et al., 2007).
Flexas et al. (2016) demonstrated that it is possible to improve WUE and crop yields by increasing photosynthesis rate (AN) accompanied with a reduction of stomatal
conductance (gs). Some possible strategies could be to raise Rubisco concentration or the
enzyme’s kinetic activity or incrementing mesophyll CO2 conductance from intercellular
space to the site of carboxylation.
The potential of this strategy, was discovered by Masle et al., (2005) in Arabidopsis
thaliana ERECTA mutants, which present a lower water loss and a higher mesophyll capacity, with a consequent increment of WUE and dry mass.
Understanding how plants regulate stomatal and mesophyllic conductance during water dehydrationcould be a potential tool for improving the WUE and photosynthesis in crops.
2.3.3 Stomatal Conductance
The stomata have a fundamental role in plant physiology and different biotechnological applications. Guard cells respond to various signals such as ABA, auxins, cytokinin, gibberellin, CO2, red and blue light and pathogens (Hetherington and Woodward, 2003).
Many studies on the manipulation of guard cells signalling components, to reduce water loss, have been already carried out (Gosti et al., 1999; Hetherington, 2001; Pei et al., 1998) and the investigation of signal transduction, involved in stomatal movements, is an interesting research topic in plant physiology (Schroeder et al., 2001).
Several MYB transcription factors gene families have an important role in the plant responses to environmental stress (Jung et al., 2007). Cominelli et al., (2010) reviewed some TFs that are involved in stomatal movements in Arabidopsis thaliana, characterizing
MYB60 as the TF for the stomatal opening process (Cominelli et al., 2005).
AtMYB60 is differently expressed in stomata cells in response to ABA, and the knock-out
mutant atmyb60-1 showed a reduction of light-induced stomatal opening and higher resistace to drought. The researchers concluded that AtMYB60 is a positive regulator of
21
stomatal opening, considering the expression is repressed during drought stress (Cominelli et al., 2005). Cominelli et al., (2005) analysed the microarray profile of the
atmyb60-1 mutant and wild-type revealing an upregulation of the delta-tonoplastic intrinsic
gene (δ-TIP) in the mutant. δ-TIP encodes for an aquaporin, suggesting the role of this transcription factor in regulating water flux in guard cells. Several genes associated with water dehydration and pathogen attack were downregulated in the atmyb60-1 mutant. Other MYB genes were being correlated to stomatal movements: AtMYB61, ATMYB44, and AtMYB15. In contrast with AtMYB60, AtMYB61 is expressed in guard cells principally in absence of light with closing stomata (Liang et al., 2005). Also, AtMYB44 and AtMYB15 are implicated in stomatal closure (Ding et al., 2009; Jung et al., 2007).
Another TF involved in the stomatal movement is NAFYA5. This TF is included in the NA-YA family group of transcription factors that binds in the cis-element CCCAT box (Gusmaroli et al., 2001). The expression is upregulated in the presence of ABA and drought and controls the stomatal aperture. NAFYA5 Arabidopsis overexpressor reduces stomatal aperture significantly under water stress, with consequently decreased water loss and improved drought resistance (Li et al., 2008).
2.3.4 Mesophyll conductance
For a long time, mesophyll conductance (gm) was considered constant and regulated by
only the leaf’s structure (Flexas et al., 2008). Centritto et al. (2003) were the first researchers to observe a significant decrease of mesophyll conductance with the intensification of water stress. Evidences suggest that environmental factors influence not only the stomata movements, but also the mesophyll conductance, mainly due to the modulation of the aquaporin and carbonic anhydrase activities (Evans et al., 2009; Flexas et al., 2008; Warren, 2008).
Aquaporins are water protein channels in the plasma and intercellular cell membranes. They are small integral membrane proteins that belong to a highly conserved family (Hachez and Chaumont, 2010; Maurel et al., 2008). Aquaporins facilitate not only water movement, but also the translocation of small molecular weight compounds, such as CO2
(Endeward et al., 2006; Yang et al., 2000). The role of aquaporins in CO2 movement are
not totally understood, but some studies suggest their involvement in the process, showed that CO2 passes through the central pore created by a tetramer of aquaporin (Wang et al.
2007). There are some evidences that aquaporins influence mesophyll conductance. In tobacco the aquaporin NtAQP1 is involved in gm in vivo: mesophyll conductance
decreased in a knock-out mutant and increased in an overexpressed mutant of Nicotiana
tabacum (Flexas et al. 2006). Moreover in Arabidopsis the deactivation of AtPIP1;2 gene,
by T-DNA insertion, showed how AtPIP1;2 is implicated in the facilitation of CO2 diffusion
(Heckwolf et al., 2011)
A second candidate for the regulation of CO2 in plants is Carbonic anhydrase (CA). The
known CA enzymes belong to three classes, α-CA, β-CA, γ-CA, and all are Zn2+
metalloenzymes, that catalyses the interconversion of CO2 to bicarbonate (HCO3-)
22
proposed that CAs facilitate the diffusion of CO2, although CA antisense mutant in tobacco
does not influence photosynthesis rate (Price et al., 1994; Williams et al., 1996). It is possible that the influence of CA in mesophyll conductance is species specific, and in plants gm is very low, because of the leaf structure (Warren, 2008). On the other hand in
Arabidopsis thaliana, there are a higher number of genes encoding for CAs. The authors
hypothesised that several different CAs located in the plasma membrane, cytosol and chloroplast could promote increased mesophyll conductance (Fabre et al. 2007).
2.4 P
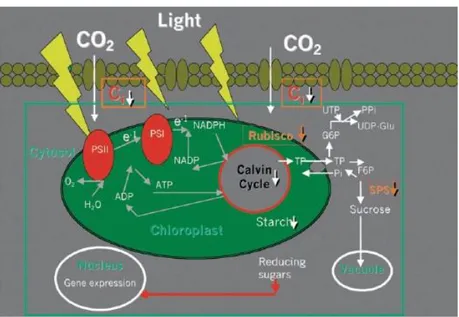
HOTOSYNTHESIS DAMAGE DURING DROUGHTPhotosynthesis is the primary process that is influenced by drought, which greatly reduces the photosynthetic capacity of plants (Chaves et al., 2009). The closure of the stomata, the defective activity of carboxylation enzymes, and the loss of photosynthetic apparatus such as ATP synthase, are the main factors that reduce carbon fixation under water scarcity (Figure 7).
Figure 7.) Under drought the declining level of intercellular CO2 concentration (Ci) decreased the activity of Calvin cycle with consequent accumulation of reducing power with the formation of ROS (see text). Also, the enzyme SPS is inhibited, causing a decrement in starch content, such as the Rubisco is de-activate due to metabolic photosynthesis impairment (Chaves and Oliveira, 2004).
The photosynthesis process involves the capture of photons by the photosystem II (PSII), which causes linear photosynthetic electron transport (PET) in the thylakoid membrane, the evolution of O2, and generation of NADPH and trans-thylakoid proton gradient (ΔpH),
which is responsible for ATP synthesis. Then the Calvin Cycle uses ATP and NADPH to synthesise Ribulose-1-5-biphosphate (RuBP), which reacts with CO2, a carboxylation
23
reaction catalysed by the enzyme Ribulose-1-5-biphosphate carboxylase/oxygenase (Rubisco) (Tezara et al., 1999).
Photosynthesis is progressively diminished under drought, but the exact mechanisms, how this happens, is unclear.
Under mild drought stress, the stomatal movement is the main limiting factor for photosynthesis. The reduced diffusion of CO2 inside the leaf and the consequent decrease
in photosynthesis rate (AN) limit the total carbon plant uptake, which can inhibits plant
growth (Chaves and Oliveira, 2004).
Limited CO2 fixation results in overreduction of the PET, which leads to photoinhibition.
Under low CO2 fixation, the reducing power rate (NADPH, NADH and ferrodoxins) is
greater than the rate of its use by the Calvin cycle (Chaves and Oliveira, 2004). The high amount of these reduced power components can promote the oxidation of molecular oxygen and generate reactive oxygen species (ROS). Inside the chloroplast, ROS negatively affects the chloroplastic ROS-responsive enzymes, changes the structure of thilakoid membrane and inhibits the traslation of Photosystem b A (PsbA) mRNA deactivating the PSII repair process (Gururani et al., 2015). During abiotic stress, the excess of reducing power rate is dissipated by three pathways: water-water cycle (WWC), photorespiration and downregulation of PSII (Asada, 2000; Miyake, 2010; Voss et al., 2013).
The WWC dissipates the excess reducing power rate using O2 as the electron acceptor
without the release of O2- and H2O2 (Asada, 2000). In WWC the superoxidase anion
radical O2 (O2-) is reduced in water, by the activity of two thylakoids enzymes. Stromal
electron acceptors such as ferredoxin (Fd), thioredoxin (TRX) and ferredoxin-NADP+
oxidoreductase, can reduce O2 to O2 superoxidase anion radical (O2-) (Foyer et al., 2012).
In WWC superoxidase anion radical is converted in H2O2 and O2 by the action of a
thylakoid membrane-bound copper/zinc superoxide dismutase (thylakoid-SOD). Thylakoid membrane-bond ascorbate peroxidases (thylakoid-APX) uses the molecule ascorbate as an electron donor to reduce H2O2 to H2O with the oxidation of ascorbate in
monodehydroascorbate (MDA) (Asada, 2000). In order to extend H2O2 scavenging, the
regeneration of ascorbate is required. The stromatal located enzyme monodehydroascorbate radical reductase (MDAR) reduces the MDA to ascorbate using NADPH as an electron donor (Miyake, 2010).
Photorespiration is the result of the oxygenase reaction of the Rubisco enzyme. The Rubisco has the dual nature of a carboxylase or oxygenase enzyme, depending on leaf CO2 or O2 concentration. At moderate water stress, Rubisco operates like oxygenase due
to a higher concentration of O2 inside the leaf, with consequent reduction of carboxylation
(Ghannoum, 2009). In the photorespiratory cycle, Rubisco catalyses the oxygenation of 1 mol of ribulose-1,5 biphosphate to produce 1 mol of 3-posphoglycerate and 1 mol of phospate. The 3-phospoglycerate feed the Calvin cycle and glycolate-2-phospate is then recycle to obatain another 0.5 mol of 3-posphoglycerate for the Calvin cycle (Wingler et al., 2000).
The photorespiration requires eight enzymes with individual reactions localised in the chloroplast, peroxisomes, mithocondrion and cytoplasm. The first step is the oxygenation of ribulose1-5-biphosphate by Rubisco. This reaction produces 3-phosphoglycerate and
2-24
phosphoglycolate (2PG). With the action of a chloroplast 2PG phospatases, 2PG is hydrolysed in glycolate. Glycolate moves out to the chloroplast and enters the peroxisome where it is converted into glyoxylate and H2O2 that is scavenged by catalases in the
peroxisome. The next step involves the glyoxylate transamination in glycine by serine-glyoxylate aminotransferase (SGT). Another parallel reaction uses glutammate from the photorespiration nitrogen cycle as the aminodonor for the transamination of glyoxylate in glycine. This reaction is performed by glutamate-glyoxylate aminotransferase (GGT). The glycine moves to the mithocondrion where it is converted in one molecule of serine, by the combinte activity of two enzymes. This reaction releases CO2 and ammonia. It has been
proposed that the CO2 relased is exported to the chloroplast in order to mitigate CO2
limitation in the Calvin cycle. Ammonia is moved to the chloroplast and refixed in glutammate by photorespiratory nitrogen cycles. The serine is then moved to peroxisome where the amino group is translocated to glyoxylate by SGT in order to produce xydroxypiruvate. A NADPH-dependent hydroxypiruvate reductase reduces the xydroxypiruvate in glycerate. The last step is the moving of glycerate in the chloroplast that is converted to 3PGA by the glycerate 3-kinase (Bauwe et al., 2010).
The process of photorespiration helps to minimise ROS production by consuming the reducing power rate ferrodoxin and NADPH in the photorespiration nitrogen cycle. Indeed, also the requirment of NADPH for hydroxypiruvate reduction would provide an additional electron sink. The NADPH can be traslocated from the chloroplast to peroxisome via the malate valve (Voss et al., 2013). However, a boosted metabolism by photorespiration requires a change in the production in chloroplast ATP relative to reductanct by the PET. The oxygenation reactions inside the chloroplast required more ATP than carbon fixation (5.375 mol ATP consumed in photorespiration vs. 3 mol of ATP in carbon fixation) (Wingler et al., 2000). In order to accommodate the alteration in chloroplast metabolism, the higher demand for ATP can be met by the PSI cyclic electron transport (PSI CET). PSI CET pathways depend only on the PSI photochemical reaction. The electron from NADPH or ferredoxin (Fd) are recycled into plastoquininone (PQ) generating ΔpH for ATP synthesis without accumulating NADPH. It has been suggested there are two main redundant pathways in PSI CET, the PGR5-dependent and the NDH complex-dipendent pathways (Shikanai, 2007). Interestingly, there is evidence that the photorespiration influences the gene expression of components involved in PSI CET such as NDH complex among
Arabidopsis thaliana grown in photorespiration conditions. These findings suggest there is
a photosynthetic control of gene expression in order to enhance the PSI CET expression that meets the ATP demand for photorespiration (Queval et al., 2012).
PSI CET has been adressed in dowregulate the PSII activity by acidifing the thylakoid lumen. Munekage et al. (2002), reported that PSI CET generates an increment of lumen acidification activating the interconversion of xanthophyll pigments. Xanthophyll are bound to the light-harvesting pigment-protein complexes (LHC) that absorb sunlight for photosynthesis (Müller et al., 2001). The xanthophyll interconversion also know xanthophyll cycle, involves the conversion of xanthophyll carotenoid violaxanthin to antheraxanthin (A) and then zeaxanthin (Z), catalysed by violaxanthin de-epoxidase (VDE) located in the lumen of thylakoid membrane (Krause and Weis, 1991). The glutamic acid-rich region of VDE can be protonated, suggesting the enzyme is active at lower pH levels
25
(Bugos and Yamamoto, 1996; Eskling et al., 1997). The zeaxanthin competes with photochemistry for absorbed light in PSII, dissipating the energy as heat. This process is also called Non-Photochemical Quenching (NPQ) (Demmig-Adams and Adams, 1996). Limited light or darkness reverses the process by epoxidation of zeaxanthin in antheraxanthin, a reaction catalysed by zeaxanthin epoxidase enzyme (ZE). To be active, this enzyme requires oxygen, NADPH and neutral pH (Gilmore, 1997). Another component involved in NPQ is the PsbS protein (Li et al., 2000). The PsbS is a Light Harvest Complex (LHC) protein. The fall in lumen pH induces a protonation of the protein causing the activation of PsbS (Li et al., 2002). More recent evidance (Correa-Galvis et al., 2016) shows that the protonation of PsbS promotes a monomerisation of dimeric PsbS with consequent interaction of PsbS with Lhcb1, the major componenet of LHC in PSII. The rearrangement of PSII is crucial for the activation of energy dissapation. The WWC discussed previously is also involved in supporting lumen acidification by proton consumption in the stroma for the photoreduction of O2 to water. Therefore, WWC allows
the ΔpH between lumen and stroma to increase, accompanied by ATP production without reductant formation (Foyer et al., 2012).
Some researchers also show the reduction of the activity of certain enzymes such as ATP synthase, sucrose-phosphate synthase (SPS) and Rubisco during drought stress (Flexas and Medrano, 2002; Maroco et al., 2002; Tezara et al., 1999).
2.5 S
ALT STRESSThe term “salt stress” usually refers to a stress induced by a high concentration of sodium chloride (NaCl), considering that NaCl is the major component in most saline soils. High salt levels induce many types of plant stress such as inhibition of potassium (K+) and
calcium (Ca+) uptake and accumulation of toxic ions such as sodium (Na+). Indeed,
osmotic and oxidative stress can occur during salt stress (Verslues et al., 2006). Salt stress differs from drought stress in the induced plant damages. In drought stress, the plant damage is triggered by soil drying, in salt stress by the accumulation of Na+ rather
than the decreased water availability (Huh et al., 2002; Munns, 2002). Drought and salt stress also differ in the gene expression profile in Arabidopsis thaliana (Seki et al. 2002). Salt stress avoidance is possible by maintaining an ion homeostasis by excluding salt from the cytoplasm, promoting salt export (Munns, 2002; Zhu, 2003). Several authors have reported that salt overly sensitive (SOS) signaling pathways play the major role in the regulation of Na+ and K+ transport between plasma membranes during salt stress (Zhu,
2002; Zhu 2003). Evidence of salt tolerance was evaluated studying the activity of the transporter HTK1 in Na+ uptake and transport inside the plant (Liu et al., 2000; Rus et al.,
2001). Other salt stress tolerance mechanisms also involve the accumulation of compatible solutes and ROS detoxification (Verslues et al., 2006).
26
It has been suggested that Na+ cross the roots’ plasma membrane cells with the activity of
channels and transporters. Calcium-permeable non-selective cation channels (NSCCs),
CYCLIC NUCLEOTIDE-GATED CHANNELS (CNGC) and GLUTAMATE-LIKE
RECEPTOR (GLR), represent the main Na+ carriers in the cells (Tester and Davenport, 2003). When plants are exposed to salt stress and in addition to the osmotic stress, the Na+ response occurs in two phases (Munns and Tester, 2008). First, Na+ exclusion is
initiated by salt stress avoidance by maintaining an ion homeostasis excluding salt from the cytoplasm roots. Several authors have reported that salt overly sensitive (SOS) signaling pathways play the major role in the regulation of Na+ transport between plasma
membranes during salt stress (Zhu, 2002; Zhu 2003). The second phase is the tolerance from an excess of Na+ in the leaves. Evidence of salt tolerance was evaluated studying the activity of the transporter in Na+ uptake and transport inside the plant (Liu et al., 2000;
Rus et al., 2001). The Na+ moved from the roots to the xylem sap due to other Na+/K+
channels and then translocated and stored in the vacuole of xylem parenchyma cells, in order to protect leaves from Na+ toxicity (Horie et al., 2005). The Histidine-tryptophan-ketoglutarate (HTK) transporters class I and II have been found to moderate Na+ transport
and Na+/K+ co-transport (Rubio et al., 1995). AtHKT1;1 gene in Arabidopsis thaliana,
encodes for an HTK class I protein involved in the removal of Na+ from the xylem sap to xylem parenchyma cells (Horie et al., 2009). Once the Na+ is inside the cytoplasm of the xylem parenchyma cells, a tonoplast carrier Na+/H+ antiporters (NHXs) imports the Na+ in
the vacuole (Apse et al., 1999). Other salt stress tolerance mechanisms also involve the accumulation of compatible solutes and ROS detoxification enzymes (Parida and Das, 2005).
2.5.1 Salt stress signaling
It is possible that plants might sense the entry of the sodium in the root cells through Na+
channels by specific sensors of Na+ (Zhu, 2003). It has been suggested that plants can
sense both separately osmotic and Na+ during salt stress. However, the idea of Na+
plasma membrane sensor, or of enzymatic Na+ sensors inside the cells such as an
osmotic sensor, is still under study (Deinlein et al., 2014; Zhu, 2003). Despite the lack of knowledge in this area, it is clear that a high concentration of NaCl induces a rapid accumulation of Ca2+ levels in the cytoplasm of roots and several cells (Kiegle et al., 2000;
Knight et al., 1997; Tracy et al., 2008). The spike of Ca2+ in the root cytoplasm triggers the
SOS signal transduction cascade, in order to protect the cell from an excess of Na+ (Ji et
al., 2013). The SOS3 gene encodes a myristoylated calcium-binding protein that functions as a primary calcium sensor to perceive the increase of Ca2+ triggered by an excess of
Na+. Upon the binding with Ca2+, SOS3 can interact and activate the serine/threonine
protein kinases SOS2, wich belongs to the SnRK3 family of protein kinases (Halfter et al., 2000; Liu et al., 2000a). The SOS3-SOS2 complex activates the Na+/H+ antiporter SOS1
with the subsequent extrusion of Na+ from the cytosol (Qiu et al., 2002; Quintero et al.,
27
The calcium spiking might also activate some calcium kinases such as Calcium-dependent protein kinases (CPKs), calcineurin B-like proteins (CBLs) with CBL-interacting protein kinases (CIPKs), which may influence the activity of several transcription factors (Boudsocq and Sheen, 2013; Weinl and Kudla, 2009). Transcription factors that are involved in salt stress response including bZIP, WRKY, AP2/ERF, MYB, bHLH and NAC families (Deinlein et al., 2014; Golldack et al., 2011). These transcription factors regulate the expression of various genes that promote the salt tolerance response in plants, such as genes relevant for inorganic ionic uptake and osmolyte biosynthesis (Geng et al., 2013). The transcription regulation of the salt-responsive genes are also mediated by the changes in hormone biosynthesis and ROS production occurring during salt stress (Deinlein et al., 2014).
28
3 O
BJECTIVE OF THE THESIS
In recent years, the use of biostimulants, often based on natural extracts, such as seaweeds, has been proposed as a sustainable strategy for improving crop yields without adversely impacting on the environment (Hernández-Herrera et al., 2014; Jayaraj et al., 2008; Khan et al., 2009).
The use of algae extracts (es. Ascophyllum nodosum) in agriculture is largely documented, as an organic and biostimulant for many varieties of crop species (Craigie, 2011; MacKinnon et al., 2010; Povero et al., 2016; Spann and Little, 2011), it was demostrated that algae extracts increase stress tolerance in treated plants (Petrozza et al., 2014; Spann and Little, 2011; Zhang and Ervin, 2004). These effects are due to a complex of bio-stimulatory components, which includes essential micronutrients, traces of vitamins, and complex organic molecules (Craigie, 2011). Notwithstanding the increasing number of papers related to this topic, the mode of action of the most part of the chemical components of these extracts remains not well characterized yet (Jithesh et al., 2012; Nair et al., 2012; Rayirath et al., 2009).
The present study was undertaken to investigate the effects of extracts, obtained from micro- and macro-algae, on the regulation of salt and water stress responses in
Arabidopsis plants, in terms of abiotic stress mitigation induced and impact on gene
expression.
The microalgae and Ascophyllum nodosum prototypes were provided by Valagro S.p.A. (Atessa, CH, Italy).
These organisms were selected to be the main compounds in the final formulation of new commercialized biostimulants, so the effects induced in the treated plants were studied in order to characterize the different products and their physiological targets.
The first step of this study was to screen different extraction methods, used for micro- and macro-algae, in order to identify the most effective one, testing plant responses. In particular different approches were used to characterize the effects of these products, starting from the study of changes induced by the treatment to the phenotype, the ability to tolerate stress condition and to the gene expression of the most affected physiologial pathways.
29
4 M
ICROALGAE EXTRACTS
4.1 M
ATERIAL ANDM
ETHODS4.1.1 Plant material and growth conditions
Arabidopsis thaliana plants, Columbia-0, treated with microalgae extracts were grown in a
Percival growth chamber (Percival scientific, IA, USA) with a photoperiod of 12-h light/12-h dark (150 µM photons m-2s-1), at 23°C day and night and 50% ± 5 relative humidity (RH).
Plants were grown in polystyrene trays floating in 1L of hydroponic solution (Table 1), as described by Gibeaut et al. (1997), in transparent plastic boxes (56x39x28 cm) changing the solution weekly. Each polystyrene plot counted 30 rock-wool plugs (Grodan, FL, USA). After sowing, the plants were incubated for two days at 4°C for vernalization and then transferred to the growth chamber. Three plots of thirty plants (ninety in total) were used for each treatment.
4.1.1.1 Microalgae extracts
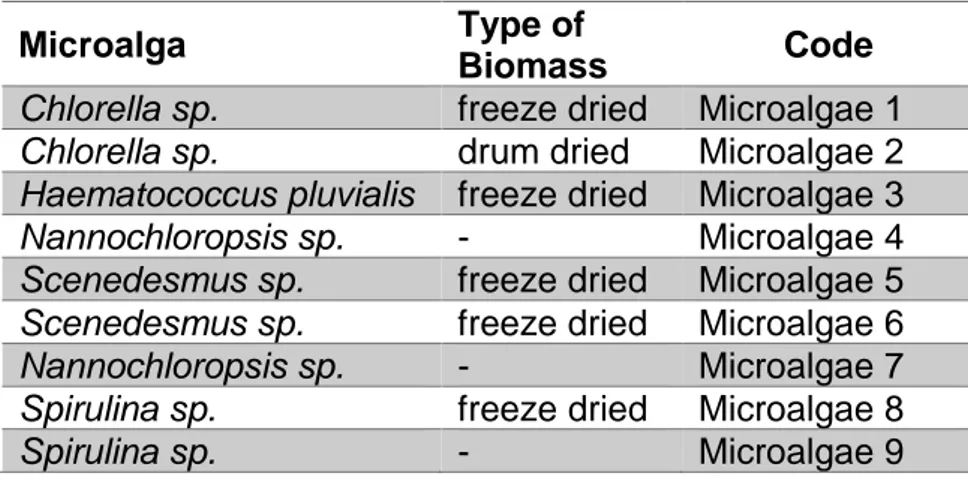
Nine microalgae extracts were used (Table 2). All the strains were provided by the company Heliae (Gilbert, AZ, USA). The microalgae were extracted by Valagro, using different procedures as described in Table 3.
30
Table 1.) Component of hydroponic solution 50 x
Group I Elements Concentration (g L-1) H3BO3 0.48 MnCl2 0.30 ZnSO4 0.087 CuCl2 0.039 (NH4)6Mo7O24 0.015 Fe-EDTA-Na 3.966 MgSO4 13.548 Group II Elements Concentration (g L-1) KCl 0.57 KNO3 18.96 KH2PO4 10.2 Group III Elements Concentration (g L-1) Ca(NO3)2 53.16
31
Table 2.) Microalgae strains used in abiotic stress experiments
Microalga Type of
Biomass Code
Chlorella sp. freeze dried Microalgae 1
Chlorella sp. drum dried Microalgae 2
Haematococcus pluvialis freeze dried Microalgae 3
Nannochloropsis sp. - Microalgae 4
Scenedesmus sp. freeze dried Microalgae 5
Scenedesmus sp. freeze dried Microalgae 6
Nannochloropsis sp. - Microalgae 7
Spirulina sp. freeze dried Microalgae 8
Spirulina sp. - Microalgae 9
Table 3.) Extraction methods used for microalgae strains
Method Description
Method N 1 extraction with two organic acids Method N 2 same as method 1 but different
temperature and time of reaction Method N 3 strong inorganic base
Method N 4 pre-extraction with an organic acid and treatment with a strong base
32
4.1.2 Drought stress
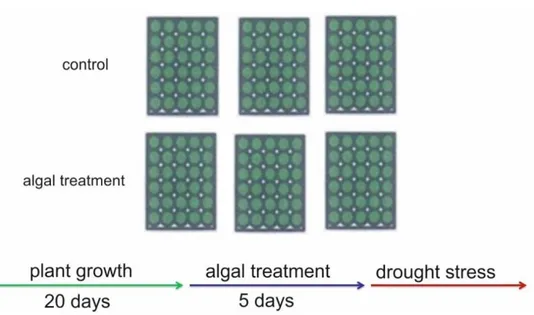
The plants were grown for twenty days in the growth chamber. To minimise the effects of any environmental gradients on plant growth, the relative position of the plastic boxes in the growth chambers were changed daily. After twenty days of growth the treatment was performed dissolving in the hydroponic medium 3 g L-1 of microalgae extracts, leaving the
trays with plants for five days in this enriched solution. As control, plants treated with the solvent of each extraction methods were used. Water stress was induced by removing the trays from hydroponic solution and keeping them at growth chamber condition. The mean of dead plants were calculated every day after dehydration (DAD) to estimate the percentage of survival and the 50% survival percentile mark, after the dehydration stress induction. Data from each treatment were compared using one-way ANOVA to detect the treatment effect (p<0.05). Data analysis was performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA).
Figure 7.) Scheme of hydroponic system used for drought stress experiments. Each green box represents a polystyrene plot with thirty Arabidopsis thaliana plants.
33
4.1.3 Salt Stress
After twenty days of growth, a first screening with with microalgae extracts method 1 was performed, dissolving in the hydroponic medium 3 g L-1 of the prototypes, and leaving the
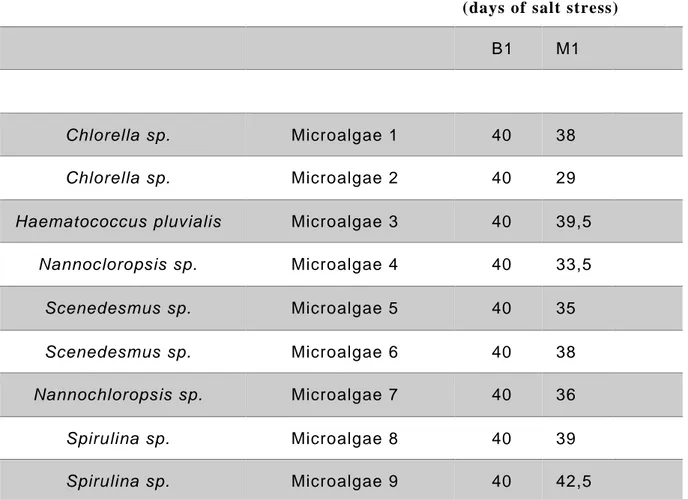
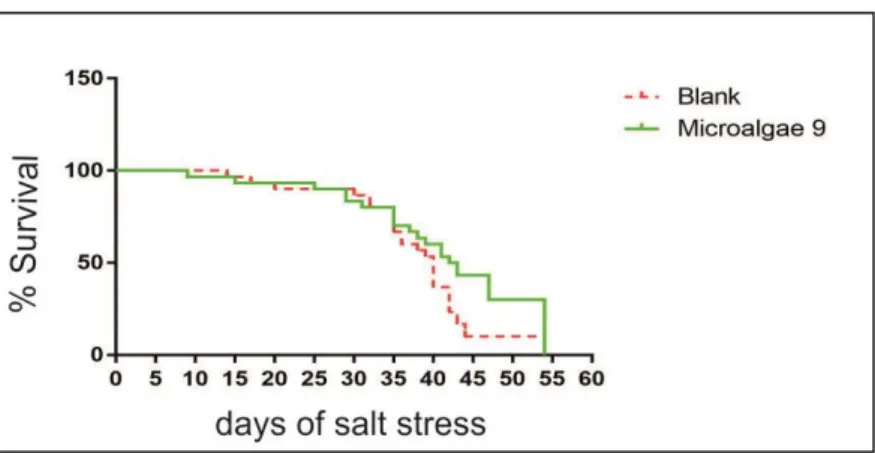
trays with plants for 5 days in this enriched solution, as represented in Figure 8. As control, plants treated with the solvent of method number 1 was used. All the polystyrene blocks were then transferred in a hydroponic solution, without any extract, in which was dissolved 100 mM of NaCl, for salt stress treatment (Figure 8). During all the days of salt stress, the percentage of survival plants was monitored and the 50% survival percentile mark were determined. The microalgae extracts, which resulted better in inducing salt mitigation, were tested, using all their different extraction methods, with 200 mM of NaCl. As control, plants treated with the solvent of each extraction methods were used (Figure 9).
The mean of dead plants were counted every day until the end of the stress, to estimate the percentage of survival and the 50% survival percentile mark. Data analysis was performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA).
34
Figure 8.) Scheme of hydroponic system used for initial pre-screening in salt stress experiments. Each green box represents a polystyrene tray with thirty Arabidopsis thaliana plants.
Figure 9.) Scheme of hydroponic system used for salt stress experiments. Each green box represents a polystyrene tray with thirty Arabidopsis thaliana plants
35
4.2 R
ESULTS4.2.1 Microalgae drought stress
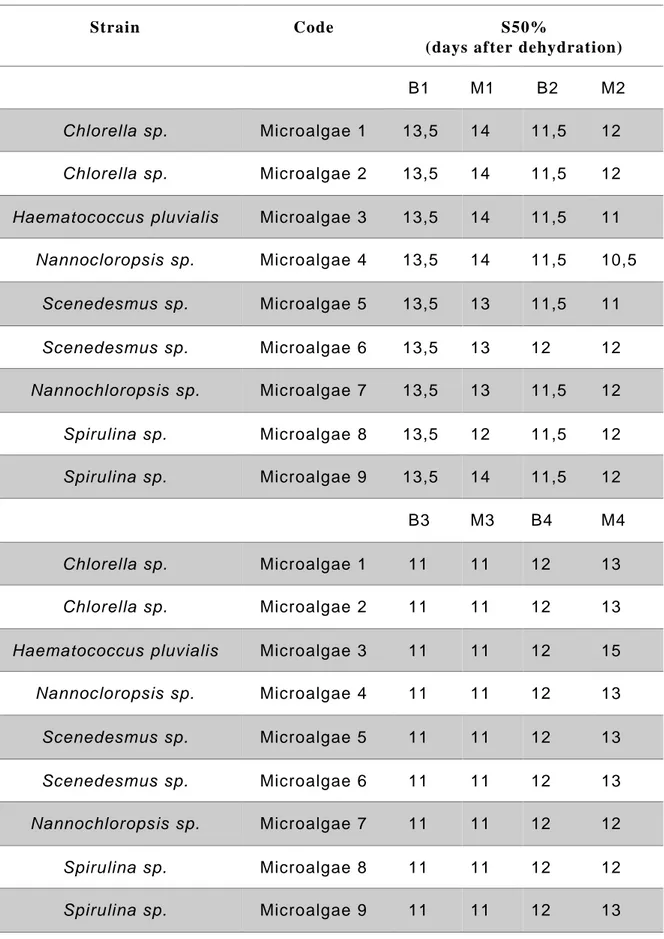
A hydroponic system was used to conduct a screening of nine microalgae strains extracted by Valagro, using four different extraction methods. Dead plants were counted over the course of the days after dehydration (DAD), from the beginning of the stress. The results of all 50% survival percentile mark (S50%) were collected and summarised in Table 4. Interestingly it was shown that the H.pluvialis (Microalgae 3), extracted with method 4 (M4), induced drought mitigation in Arabidopsis thaliana plants (Table 4 and Figure 10). Survival analysis showed that the A.thaliana treated with H.pluvialis M4 reach the S50% at 15 DAD, while in control 12 DAD (Table 4). The survival graph (Figure 10) showed that the treatment (green line) increase the mitigation to the stress compared to the untreated control (red line). This can be highlighted in Figure 10.b where the number of alive plants, over the course of the 18-days after dehydration period, was higher in the treated plants.
Overall, these data showed that H.pluvialis (Microalgae 3) extracted with method 4 (M4) increases drought stress mitigation in Arabidopsis thaliana plants.
No substantial differences were found with other microalgae strains, regardless of the extraction method used.