THE
JOURNAL • RESEARCH • www.fasebj.org
A novel 3
9-tRNA
Glu
-derived fragment acts as a
tumor-suppressor in breast cancer by
targeting nucleolin
Maurizio Falconi,*,1,2Mara Giangrossi,*,1 Maria Elexpuru Zabaleta,*,1Junbiao Wang,* Valentina Gambini,* Martina Tilio,* Daniela Bencardino,* Sergio Occhipinti,† Barbara Belletti,‡Emiliano Laudadio,§
Roberta Galeazzi,{Cristina Marchini,*,1,3and Augusto Amici*,1
*School of Biosciences and Veterinary Medicine, University of Camerino, Camerino, Italy;†Department of Molecular Biotechnology and Health Sciences, Center for Experimental Research and Medical Studies, University of Torino, Torino, Italy;‡Division of Molecular Oncology, Centro di Riferimento Oncologico di Aviano (CRO) Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), National Cancer Institute, Aviano, Italy; and§Dipartimento Scienze e Ingegneria della Materia, dell’Ambiente ed Urbanistica, and{Dipartimento di Scienze della Vita e dell’Ambiente, Universit`a Politecnica delle Marche, Ancona, Italy
ABSTRACT:tRNA-derived fragments (tRFs) have been defined as a novel class of small noncoding RNAs. tRFs have been reported to be deregulated in cancer, but their biologic function remains to be fully understood. We have identified a new tRF (named tRF3E), derived from mature tRNAGlu, that is specifically expressed in healthy mam-mary glands but not in breast cancer (BC). Consistently, tRF3E levels significantly decrease in the blood of patients with epidermal growth factor receptor 2 (HER2)-positive BC reflecting tumor status (control> early cancer > meta-static cancer). tRF3E down-regulation was recapitulated inD16HER2 transgenic mice, representing a BC preclinical model. Pulldown assays, used to search for proteins capable to selectively bind tRF3E, have shown that this tRF specifically interacts with nucleolin (NCL), an RNA-binding protein overexpressed in BC and able to repress the translation of p53 mRNA. The binding properties of NCL-tRF3E complex, predictedin silico and analyzed by EMSA assays, are congruent with a competitive displacement of p53 mRNA by tRF3E, leading to an increased p53 ex-pression and consequently to a modulation of cancer cell growth. Here, we provide evidence that tRF3E plays an important role in the pathogenesis of BC displaying tumor-suppressor functions through a NCL-mediated mechanism.—Falconi, M., Giangrossi, M., Elexpuru Zabaleta, M., Wang, J., Gambini, V., Tilio, M., Bencardino, D., Occhipinti, S., Belletti, B., Laudadio, E., Galeazzi, R., Marchini, C., Amici, A. A novel 39-tRNAGlu-derived
frag-ment acts as a tumor-suppressor in breast cancer by targeting nucleolin. FASEB J. 33, 000–000 (2019). www.fasebj.org
KEY WORDS:RNA-protein interaction • small noncoding RNAs • p53 protein • HER2
Breast cancer (BC) is the second cause of cancer-related deaths in women. Epidermal growth factor receptor 2 (HER2)-positive BC accounts for about 20% of all BC cases,
and it is one of the most aggressive subtypes (1). A deeper knowledge of the relevant molecular mechanisms behind the onset and progression of BC is needed in order to identify new markers and develop more effective thera-pies. Over the last 2 decades, massive amounts of small noncoding RNAs (ncRNAs) with regulatory functions have been discovered, and increasing evidence has dem-onstrated that ncRNAs are aberrantly expressed in BC, emerging as promising biomarkers for diagnosis, prog-nosis, and prediction of response to therapy (2, 3). Cur-rently, the new class of tRNA-derived fragments (tRFs) has been recognized to be the major RNA species in human cells playing a regulatory role in several biologic processes (4–6). For a long time, tRFs were considered as non-functional products of random tRNA cleavage or degra-dation. Now the biogenesis of tRFs is under investigation and there is some evidence that, although tRFs are het-erogeneous in size (14–45 nt), their ends are precisely
ABBREVIATIONS:Akt, protein kinase B; BC, breast cancer; FBS, fetal bo-vine serum; FVB mouse, inbred strain susceptible to the Friend leukemia virus B; HER2, epidermal growth factor receptor 2; miRNA, microRNA; MM-PBSA, Molecular Mechanics–Poisson Boltzmann Surface Area; NCL, nucleolin; ncRNA, noncoding RNA; PDB, Protein Data Bank; pre-rRNA, preribosomal RNA; RBD, RNA-binding domain; qRT-PCR, quantitative RT-PCR; tRF, tRNA-derived fragment; WT, wild-type
1These authors contributed equally to this work.
2Correspondence: School of Biosciences and Veterinary Medicine, Uni-versity of Camerino, via Gentile III Da Varano, 62032 Camerino, Italy. E-mail: [email protected]
3Correspondence: School of Biosciences and Veterinary Medicine, Uni-versity of Camerino, via Gentile III Da Varano, 62032 Camerino, Italy. E-mail: [email protected]
doi: 10.1096/fj.201900382RR
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
defined by RNA cleavage sequence determinants and their formation is regulated. In particular, tRFs derived from mature tRNA can be divided into 2 major types: tRNA halves, cleaved at the anticodon loop by angiogenin, and smaller tRFs: 59- 39-tRFs, cleaved at the TCC and D loop by Dicer (7). Several studies have demonstrated that tRFs are dysregulated in cancer and may have oncogenic or tumor-suppressor functions. Although accumulating evidence suggests that tRFs may behave like microRNAs (miRNAs) targeting the 39UTR of specific mRNAs and repressing their translation (8), each tRF reasonably may adopt its individual mode of action. Importantly, tRFs have been also found as circulating species in a wide va-riety of biologic fluids either within exosomes or outside these microvesicles, associated to Argonaute proteins (9). Intriguingly, RNA packaging within exosomes is not random but selective because small ncRNAs content dif-fers between cancer and parental cells strengthening the idea that exosomes with their RNAs cargo play a key role in the tumorigenic process (10).
In this study, we identified a novel circulating tRF (named tRF3E) matching the 39 half-molecule of mature tRNAGluTTC. By comparing the levels of tRF3E in blood samples from patients with HER2-positive BC and healthy individuals, we found that tRF3E expression significantly decreased in BC reflecting tumor status (control. early cancer . metastatic cancer), thus indicating that tRF3E plays an important role in the pathogenesis of this disease. By using tRF3E as bait in a pulldown assay, nucleolin (NCL) was identified as the main protein able to specifi-cally interact with tRF3E.
NCL is a multifunctional protein abundantly expressed in the nucleolus, where it controls early steps of ribosome biogenesis, but it is also found in the nucleoplasm and the cytoplasm as well as on the cell membrane, where it modulates cell proliferation, survival, and apoptosis (11, 12). The overexpression of NCL has been detected in many different human cancers, including breast tumors (13), and it is associated with poor prognosis, particularly when the cytoplasmic fraction of NCL is increased (14). Certainly, the great versatility of NCL resides in its ability to interact with both nucleic acids and proteins. In fact, NCL is
formed by 3 structural domains endowed with distinct functions. The N- and C-terminal regions are required for protein-protein interactions, whereas the central domain contains 4 RNA-binding motifs that mediate its interaction with target RNAs. Thus, NCL can modulate at the post-transcriptional level the fate of specific mRNAs affecting their stability or translation (15, 16).
Here, we demonstrate that tRF3E has tumor-suppressor functions, and we provide mechanistic evidence that tRF3E, by competitive interaction with NCL, causes the release of p53 mRNA, a known messenger bound by NCL (17, 18), thereby promoting its translation.
MATERIALS AND METHODS
General procedures
DNA and RNA samples were quantified with NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA). Radioactive bands on solid supports were detected and quantified by Mo-lecular Imager (model FX; Bio-Rad, Hercules, CA, USA). Purified human NCL was provided by MyBioSource (San Diego, CA, USA). This protein is missing the N-terminal region, and its molecular mass is 55 vs. 70 kDa of wild-type (WT) protein. DNA and RNA oligonucleotides are listed in Table 1. Databases used in this study were: miRBase (http://www.mirbase.org), piwiRNA (www.pirnabank.ibab.ac.in), NCBI BLAST (www.blast.ncbi.nlm.nih.gov), Genomic tRNA (http://gtrnadb.ucsc.edu/GtRNAdb2/blast.html), Dicer Cleavage Sites (http://crdd.osdd.net/raghava/phdcleav/submission.html), and Protein Data Bank (www.rcsb.org).
Human specimens
Patients with BC were recruited at the Centro Oncologico Ema-tologico Subalpino (COES), Azienda Ospedaliero-Universitaria (AOU) Citt`a della Salute e della Scienza di Torino (Torino, Italy), with informed consent. Samples from healthy subjects were provided by the local Torino Cord Blood Bank (Torino, Italy). Plasma obtained from heparinized venous blood samples by centrifugation were immediately frozen at280°C. The human studies were conducted according to the Declaration of Helsinki principles. Human investigations were performed after approval of the study by the Scientific Ethics Committee of AOU Citt`a della TABLE 1. DNA and RNA oligonucleotides used
Name Primer length (mer) Sequence, 59–39
mirR1 20 AUGUCAAGCUUAUAACCGAA mirD1H 20 ATGTCAAGCTTATAACCGAA tRF5E-M 18 TCCCACATGGTCTAGCGG tRF3E-MRC 25 CACCGGGAGTCGAACCCGGGCCGCC dT20B 31 CGCGGATCCTTTTTTTTTTTTTTTTTTTTVN tRF3E-M 20 AGGCGGCCCGGGTTCGACTC GR49-80 23 ACGATATATGCAAAAACATATTA RNAG49-80 (M1) 32 ACGAUAUAUGCAAAAACAUAUUAAACAAAGCC M2 66 TTCGCATGCTTCAAATATGTATCCGCTCATGAT-ACAATTCTCCCGTTGCATTGATATATAACACAG tRF3E 32 AGGCGGCCCGGGUUCGACUCCCGGUGUGGGAA tRF3E-MB 32 AGGCGGCCCGGGUUCGACUCCCGGUGUGGGAA/3Bio/ CR2 20 AUGUCAAGCUUAUAACCGAA Mol130 24 UGCUCUAGAUAAAGACUAGAGCUU
Salute e della Scienza di Torino (Protocols 0085724 and 0012068). Written informed consent was received from each participant before inclusion in the study and specimens were identified be-fore analysis. Small RNA extraction from human plasma was performed by NucleoSpin miRNA Plasma kit according to the manufacturer’s protocol (Macherey-Nagel, D¨uren, Germany). Mice
D16HER2 and WT FVB mice [FVB (inbred strain susceptible to the Friend leukemia virus B)/NCrl (mouse strain from Charles River Laboratories, Wilmington, MA, USA)] were housed under controlled temperature (20°C) and circadian cycle (12-h light/ 12-h dark) in the animal facility of University of Camerino. D16HER2 transgenic and WT FVB mice were fed a chow diet (Mucedola, Settimo Milanese, Italy) and tap water ad libitum. Female WT FVB mice were used in all experiments to match tumor-prone D16HER2 female mice according to genetic back-ground and sex. D16HER2 transgenic females were monitored weekly by palpation to assess tumor onset. Tumor diameter was measured by digital caliper. Masses.2 mm in mean diameter were regarded as tumors. Blood was collected from the orbital sinus under anesthesia. In order to collect serum, whole blood samples were left to clot at room temperature for 20 min. Serum separation was accomplished by 2 subsequent centrifugations at 2000 g at 4°C. All animal experiments were carried out in ac-cordance with the United Kingdom. Animals (Scientific Proce-dures) Act, 1986, and associated guidelines, European Union Directive 2010/63/EU for animal experiments. All the proce-dures were approved by the Ethic Committee on Animal Use of the University of Camerino (Protocol 14/2012).
Cloning strategy of tRF3E
Exosomes from mouse serum were isolated by ExoQuick kit (System Biosciences, Palo Alto, CA, USA) and used for RNA extraction by TriFast reagent according to the manufacturer’s protocol (EuroClone, Pero, Italy). Then, 100 ng of total RNA were polyadenylated at 39-ends using 2.5 U of Escherichia coli Poly(A) Polymerase (New England Biolabs, Ipswich, MA, USA) at 37°C for 30 min; after ethanol precipitation, the 59-ends of RNA were ligated to the RNA adapter mirR1 (200 pmol) using 10 U of T4 RNA Ligase 1 (New England Biolabs) in 20 ml of reaction mixture containing 1 mM ATP. After an incubation of 2 h at 37°C, RNA was phenol-chloroform (1:1) extracted and precipitated with ethanol. After heating the sample at 65°C for 10 min, the RNA primer was extended at 42°C for 60 min in a reaction mixture containing 0.5 mM of the 4 deoxynucleotides, 10 U of AMV Re-verse Transcriptase (New England Biolabs) and 100 pmol of dT20B oligonucleotide as primer. The resulting cDNA was am-plified by PCR using the Perfect Taq Polymerase Kit (5Prime; Thermo Fisher Scientific) and the primers mirD1H and dT20B, containing at their 59-ends the HindIII and BamHI sites, re-spectively. The amplified DNA fragments were inserted into the BamHI and HindIII sites of PTZ19R (MilliporeSigma, Burlington, MA, USA) plasmid, and the ligation products were then trans-formed into the E. coli DH5a strain. After colony screening by PCR, the plasmid DNA was purified by the NucleoSpin Plasmid QuickPure Kit (Macherey-Nagel) and DNA sequencing (BMR Genomics, Padua, Italy).
Cloning strategy of tRF5E
cDNA derived from WT FVB mouse was amplified with dT20B and the specific primer tRF5E-M. The amplicon, after a blunting enzyme treatment, was inserted in 1.2/blunt plasmid using
CloneJet PCR Cloning Kit (Thermo Fisher Scientific). Ligation products were then used to transform E. coli Top10 competent cells and recombinant plasmids were purified and sequenced as in-dicated above.
Electroblotting
The RNA samples were resuspended in loading buffer (95% formamide, EDTA 10 mM, bromophenol-blu, and xylene cyanol 0.01%), denaturated at 65°C for 5 min, loaded on 8% PAGE-urea gel in Tris-borate EDTA buffer 0.5X and transferred to a neutral nylon Hybond-N membrane (Amersham, Little Chalfont, United Kingdom) by semidry electroblotting (Trans-Blot Turbo Transfer System; Bio-Rad) at 0.3 A for 30 min. The membrane was hy-bridized with the [32P]-labeled tRF3E-MRC probe.
Semiquantitative RT-PCR
Semiquantitative RT-PCR was performed on cDNA derived from 10 ng of polyadenylated RNA extracted from serum using the indicated specific primer for tRF3E coupled to dT20B oligo-nucleotide and the Perfect Taq polymerase Kit (5Prime). We applied a limited number of cycles (32–34), experimentally de-termined, so that the PCR reaction proceeded linearly as a func-tion of cDNA template and the amplificafunc-tion product proportionally reflected the initial amount of tRF3E present in the serum.
Quantitative RT-PCR
Quantitative PCRs (qPCRs) were performed on Mx3000P QPCR Systems (Agilent Technologies, Santa Clara, CA, USA; Stra-tagene, San Diego, CA, USA) in 96-well plates (PCR Microplate; Corning, Corning, NY, USA) using SYBRPremix Ex Taq II Kit (Takara, Kyoto, Japan). Each individual quantitative (q)RT-PCR assay was performed in a final volume of 25 ml using 5 ng of cDNA as template, the common primer dT20B and the specific primers GR49-80 or tRF3E-M for spike-in or tRF3E amplification, respectively. Absolute quantification of tRF3E by PCR was obtained generating in parallel a standard curve containing from 13 106to 53 109copies of plasmid pTZ19R carrying tRF3E DNA and using the primers dT20B and tRF3E-M. Data analysis for qRT-PCR was performed using MxPro QPCR Software (Agilent).
Pulldown assay
Pulldown experiments were performed using streptavidin-agarose resin (Thermo Fisher Scientific). After equilibrating the streptavidin-agarose resin (80 ml) with buffer base A [10 mM Tris-HCl pH 7.5, 100 mM NaCl, 1 mM EDTA, 0.1% Nonidet P-40 (MilliporeSigma)], 3 mmol of the biotinylated tRF3E oligonucle-otide (tRF3E-MB) were ligated to the resin at room temperature for 1 h in 700 ml of the same buffer. Then, the resin was washed twice with 700 ml of buffer base A supplemented with 750 mM NaCl and once with the same buffer containing 150 mM NaCl before adding 480 mg of SK-BR-3 cells’ protein extract. The binding of proteins to the immobilized RNA was obtained by incubation for 2 h at 4°C. Then, the resin was washed 4 times with 500 ml of buffer base A containing increasing concentrations of NaCl (100–500 mM). Finally, proteins bound to the tRF3E were removed by incubation with 120 ml of elution buffer (10 mM Tris-HCl pH 7.5, 1 mM Na-EDTA, 2% SDS) at room temperature for 5 min. The proteins eluted from several pulldown experi-ments (;12) have been pooled together and resolved in
SDS-PAGE followed by mass spectrometry analysis (Proteomics Facility Unit, University of Chieti, Chieti, Italy).
Western blot
Proteins derived from pulldown experiment or cell lysates, obtained using RIPA buffer (1% Nonidet P40, 0.5% Na-deoxycolic acid and 0.1% SDS in PBS) with protease in-hibitors, were subjected to Western blot analysis as described by Andreani et al. (19). The antibodies to NCL (sc-8031; 1:1000), b-actin (sc-47778; 1:1000), protein kinase B (Akt) (9272, 1:1000; Cell Signaling Technology, Danvers, MA, USA), pAkt (4060, 1:1000; Cell Signaling Technology), p53 (sc-6243, 1:800; Santa Cruz Biotechnology, Dallas, TX, USA), and p21 (OP64, 1:250; MilliporeSigma) were used. Mouse or rabbit horseradish peroxide–conjugated secondary antibodies were used. Filters were incubated with LiteAblot Turbo (EuroClone) and the immunoreactive proteins were detected with ChemiDoc XRS-System (Bio-Rad).
EMSA
Oligonucleotide tRF3E was [32P]-labeled using T4 polynucleotide kinase. EMSA was made in 15 ml of Gel Retardation buffer (20 mM Tris-HCl, pH 7.5, 50 mM KCl, 10% glycerol, 0.3 mg/ml bovine serum albumin, 0.02% Nonidet P-40) incubating 0.25 pmol of32[P]-tRF3E with increasing amounts of NCL at 23°C for
20 min. The competitive EMSA was performed under the same experimental conditions preincubating 0.25 pmol of [32P]-tRF3E
with a fixed concentration of NCL before adding increasing amounts of unlabeled RNAs as indicated. Then the incubation was prolonged for additional 30 min. Samples were loaded on native 10% polyacrylamide gel.
Cell culture
SK-BR-3 and Michigan Cancer Foundation (MCF)7 human BC cells were obtained from American Type Culture Collection (Rockville, MD, USA) and cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (Thermo Fisher Scientific). Cellular automata ma-chine (CAM)6 cells, a D16HER2-expressing epithelial tumor cell line derived from a mammary carcinoma spontaneously arisen in a D16HER2 female mouse (20), were maintained in DMEM supplemented with 20% FBS and 1% penicillin-streptomycin. Cells were grown in a humidified atmosphere with 5% CO2at
37°C.
Cell transfection and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
MCF7 cells were seeded in 96-well plates (13 104cells/well) 24 h before transfection by using a medium without antibiotics. Lip-ofectamine 3000 (Thermo Fisher Scientific) was used as trans-fection reagent following the manufacturer’s instructions. After 24 h of incubation, cells were transiently transfected in OptiMEM (Thermo Fisher Scientific) with 200 nM of tRF3E (Intergrated DNA Technologies (IDT)) or CR2 RNA as control. For this pur-pose, RNA/lipid complexes were prepared in OptiMEM and left for 20 min at room temperature before adding them to the cells. After 4 h of incubation at 37°C, complete medium was added into each well. Effects of tRF3E on MCF7 cell viability were evaluated 48 h after transfection by means of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described in Kalogris et al. (21). Experiments were performed in octuplicate and
repeated 3 times. Quantitative data are presented as means6SD.
The significance of differences was evaluated with 1-way ANOVA. Statistical analysis was carried out with Prism 5 Software (Graph-Pad Software, La Jolla, CA, USA). A value of P# 0.05 was used as the critical level of significance.
Immunofluorescence analysis
SK-BR-3 cells were plated on glass coverslips, previously coated with FBS for 30 min, in a 12-well plate. One day after plating, 70–90% confluent cells were transfected with biotinylated-tRF3E-MB (IDT) using Lipofectamine 3000, according to the manufac-turer’s instructions. After 6 h, cells were washed, fixed with cold 100% methanol for 5 min, and permeabilized with 0.5% Triton X-100 for 7 min before incubation in 10% bovine serum albumin for 20 min at room temperature. Then, cells were incubated with Cy3-conjugated IgG Fraction Monoclonal Mouse Anti-Biotin (1:400; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at 37°C for 1 h in the darkness before a further incubation with 0.1 mg/ml DAPI for 10 min. Finally, cells were examined under LSM 510 Meta Laser Scanning Fluorescence Confocal Microscope (Carl Zeiss GmbH, Oberkochen, Germany). Computational modeling of NCL
The complete structure of NCL was reconstructed starting from the Protein Data Bank (PDB) fragments previously crystallized (PDB codes 2KRR, 2FG9, 2FG8), corresponding to the RNA-binding domains (RBDs) and using Iterative Threading ASSEmbly Refinement (I-TASSER) (22). In addition, ModLoop (23) and GalaxyLoop (24) were used for loops modeling and refinement. NCL model was created in a simulation box of 153 153 15 nm, adding TIP3P water molecules and NaCl, to reach the physiologic conditions taking into account the net charge of NCL protein. The protein was minimized with 10,000 cycles steepest descent followed by 5000 steps conjugate gradient, obtaining a convergence of maximum force to energy threshold of 1000 kJ/mol nm2. Then, 6 equilibration steps let NCL grad-ually accommodate within the aqueous environment; the Verlet cutoff scheme for neighbor searching, combined with particle mesh Ewald (PME) for electrostatics, was used. The cutoff for the Van der Waals forces calculation was settled to 1.2 nm with force smoothly switched to 0 (between 1.0 and 1.2 nm) generating the velocities at 310 K in NVT ensemble using a Maxwell distribution function with random seed (Berendsen thermostat) (2 simulation runs, 25 ps). Then, we shifted to the NPT ensemble maintaining the weak coupling also for pressure control (Berendsen barostat, isotropic conditions, 1 bar, time coupling 5 ps), maintained for 4 equilibration runs (50 ps). In the 20 ns production phase, we shifted to Nos´e-Hoover and Parrinello-Rhaman algorithms for temperature control and pressure coupling, respectively; leapfrog algorithm and a time step of 0.002 ps were used. On the obtained trajectories, we calculated the Molecular Mechanics–Poisson Boltzmann Sur-face Area (MM-PBSA) energies of all NCL-RNAs systems shown in Supplemental Fig. S6. AMBER99-SB-ILDN force field as implemented in GROMACS 5.0.4 software package was used (25).
Statistical analysis
Quantitative data are presented as means 6 SEM from 3
in-dependent experiments. The significance of differences was evaluated with a 2-tailed Student’s t test or 1-way ANOVA. Sta-tistical analysis was carried out with GraphPad Prism 5 Software. A value of P# 0.05 was used as the critical level of significance.
RESULTS
Identification of a circulatingtRFGluTTC
The present study was initially focused on the identifica-tion of new circulating small ncRNAs in mouse. Shortly, small RNAs, extracted from serum exosomes of WT FVB mouse, were polyadenylated adding a polyA tail at 39-end and ligated to an RNA oligonucleotide adapter at 59-ter-minus. The resulting modified RNAs were reverse tran-scribed using a poly-dT primer to obtain cDNAs that were amplified by PCR, cloned into PTZ19R vector, and ana-lyzed by DNA sequencing. This molecular strategy allowed us to identify a 32-nt-long DNA sequence corre-sponding to the following RNA: 59-AGGCGGCCCGG-GUUCGACUCCCGGUGUGGGAA-39. To know the nature of this small RNA, we first analyzed it by aligning its 32 nt sequence with those contained in 2 different databases: miRBase and piwiRNA database. However, this research was unsuccessful. Thus, the 32-nt RNA sequence was queried against the U.S. National Center for Biotechnology Information (NCBI) BLAST database (www.blast.ncbi.nlm.nih.gov). Interestingly, a complete matching was found at multiple loci within tRNAGluTTCsuggesting that this RNA could belong to the class of tRFs. This hy-pothesis was confirmed by aligning this putative tRF with the tRNA gene sequences from mouse (the source organism) and humans, contained in the Genomic tRNA database. Hence, we discovered a new tRF, named tRF3E, that corresponds to the whole TCC-loop of tRNAGluTTC, extending from position 41 to its 39-terminus at position 72 (Fig. 1A). Given that cloning fre-quency approximately reflects the relative quantity of a given RNA species, this tRF3E is expected to be particu-larly abundant within exosomes of WT FVB mice.
Angiogenin and Dicer are the main RNases responsible for tRF production (7). Angiogenin produces a specific cleavage at the anticodon loop to generate 59- and 39-tRNA halves. Because the size and, in particular, the 59-end of tRF3E were not compatible with an Angiogenin-mediated processing, the sequence of mature tRNAGluTTCwas analyzed in silico (Prediction of Human Dicer Cleavage) to predict Dicer cleavage sites. This analysis shows that Dicer can generate a 59-tRF and a 39-tRF of 31 and 32 nt, respectively. The 32-nt fragment, corresponding to the 39-end of the parent tRNAGlu, matches perfectly with the tRF3E identified in the present study (Fig. 1A). By adopting a cloning strategy analogous to that described for the tRF3E, we also found the 31-nt fragment, corre-sponding to the predicted 59-tRF generated by Dicer (called tRF5E), in mouse exosomes.
To corroborate tRF3E existence in the bloodstream, we also performed a northern analysis, demonstrating that tRF3E was present in mouse serum RNA (Fig. 1B) and became even more evident as a RT-PCR amplification product when the same RNA was used as template. Of note, CAM6 tumor cells, the in vitro counterpart of D16HER2 mammary tumors, contained only the full-length tRNAGlu, whereas they did not express detectable levels of tRF3E, suggesting that tRF3E generation could be reduced or even totally suppressed in BC. Finally, we
verified by RT-PCR that tRF3E was released even in hu-man blood, where instead the full-length tRNAGluTTCwas absent (Supplemental Fig. S1).
tRF3E decreases in BC
Because it has been reported that tumor induces changes in the abundance and nature of circulating small ncRNAs, we evaluated whether tRF3E serum level varied during breast carcinogenesis in D16HER2 mice. D16HER2 trans-genic mice have been chosen as a preclinical model of mammary tumorigenesis because they develop fast-growing tumors due to the expression of the D16HER2 oncogenic splicing variant in the mammary gland. In
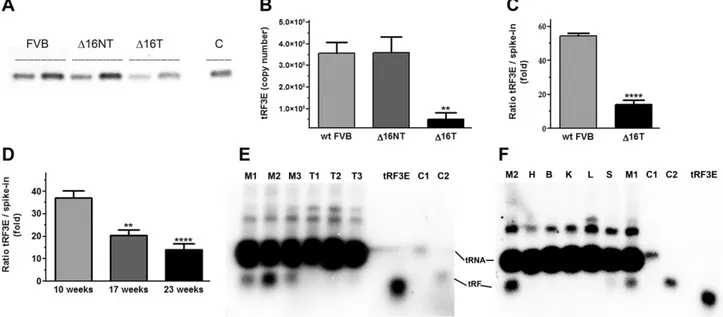
Figure 1. Identification of tRF3E in sera of WT FVB mice. A) 59-tRF and 39-59-tRF are indicated with dashed and solid lines, respectively, on the structure of tRNAGlucloverleaf. The in silico prediction of Dicer cleavage on the tRNAGludisplays 2 cuts at nt 31 and 40 (arrows), giving rise to a 59-tRF and a 39-tRF of 31 and 32 nt, respectively. B) Electroblotting was performed as described in Materials and Methods and filter hybridized with the [32 P]-labeled tRF3E-MRC oligonucleotide. Lane 1 contains 1 mg of total RNA from CAM6 cells. Lanes 2 and 3 show the levels of tRF3E (32 nt) in 500 and 300 ng of serum RNA from WT FVB mice, respectively. In lanes 4 and 5, the PCR or RT-PCR amplification products of tRF3E (63 nt) and full-length tRNAGlu(103 nt) are loaded, respectively. These amplicons were obtained with dT20B as common reverse primer and tRF3E-M as forward primer for tRF3E (amplicon from the original PTZ19R clone) or tRF5E-M for full-length tRNAGlu(using;2 ng of total polyadenylated RNA
from CAM6 cells as template). The oligonucleotides M1 (32 nt) and M2 (66 nt) were used as markers.
particular, all D16HER2 female mice develop multiple mammary tumors between 12 and 19 wk of age, with a mean latency of about 15 wk (20, 26–28). As shown in Fig. 2A, the serum level of tRF3E, analyzed by semi-quantitative RT-PCR, is comparable between WT FVB and D16HER2 mice before tumor onset (D16NT), whereas it is clearly reduced in tumor-bearing D16HER2 mice (D16T). This result was confirmed by measuring the absolute number of tRF3E molecules, which was much lower (;7-fold) in the serum of D16HER2 mice that had palpable tumors with respect to tumor-free D16HER2 and WT FVB mice (Fig. 2B). To normalize the tRF3E copy number in the absence of a reference gene, a synthetic spike-in RNA (32 nt) was added to the serum as an external control before RNA extraction, and it was quantified by qRT-PCR in parallel with tRF3E, as already described in other studies related to serum biomarker identification (29, 30). This new analysis reveals again a significant drop (;3.5-fold) of tRF3E serum concentration in tumor-bearing D16HER2 mice with respect to WT FVB healthy mice (Fig. 2C). To further prove this inverse correlation between tRF3E levels and tumor progression, we analyzed the concentration of tRF3E in the serum of D16HER2 mice at different stages of
tumor development. In particular, taking into account the kinetic of BC growth in these transgenic animals, we measured tRF3E in the blood collected from the same mice at 3 different times: at 10 wk of age, when mice were still tumor free; at 17 wk of age, when mice started to develop a small palpable mass; and finally at 23 wk of age, when all the mice had multiple mammary carcinomas. As shown in Fig. 2D, the level of tRF3E gradually decreases from the 10th to the 23rd weeks of age, while during the same time period the tumor burden increases. To investigate if the observed reduction of circulating tRF3E was due to a de-regulation of its biogenesis in BC, we analyzed the ex-pression of tRF3E vs. mature tRNAGlu in tumors and normal mammary glands by northern blot. As shown in Fig. 2E, tRF3E is clearly detectable in mammary glands explanted from WT FVB mice, but it is not visible in mammary adenocarcinomas excised from D16HER2 mice. Quantification of the spots corresponding to tRF3E and tRNAGlu(lanes M1-M3 in Fig. 2E, F) reveals that tRF3E is relatively abundant, ranging between 2.4 and 4.7% of the mature tRNAGlu. In particular, we estimated that mam-mary gland cells contain on average;80,000 tRF3E mol-ecules per cell when we assume a general amount of 10 pg
Figure 2. BC causes a decrease of tRF3E in mouse serum. A) Semiquantitative RT-PCR was performed starting from polyadenylated RNA extracted from serum of WT FVB and D16HER2 transgenic mice before tumor development (D16NT) and with evident tumor masses (D16T). The tRF3E-M and dT20B primers were used (Table 1). Two different volumes (2.5 and 5 ml) of each sample were loaded on agarose gel, and C is the positive control for tRF3E (amplicon from the original PTZ19R clone). B) Absolute quantification of tRF3E was carried out by qRT-PCR (see Materials and Methods). In all experiments, values are means 6SEM, n = 4. **P = 0.003 (D16T was compared with FVB), **P = 0.0054 (D16T was compared with D16NT). Statistical
analysis was calculated with ANOVA plus Bonferroni post hoc tests. C ) tRF3E level in WT FVB and D16HER2 transgenic mice at 23 wk of life. ****P, 0.0001 (D16T was compared with WT FVB by Student’s t test). D) Relative qRT-PCR quantification of tRF3E vs. spike-in RNA in D16HER2 transgenic mice during tumor development. Statistical analysis was calculated with ANOVA plus Bonferroni post hoc tests. Each group was compared to control (10-wk-old mice). **P = 0.0021, ****P, 0.0001. E, F) Northern blot analysis of full-length tRNAGluand tRF3E was performed on total RNA, extracted from different normal tissues and tumors. RNA (10 mg) was run on a denaturing 4% high resolution agarose gel, and membranes were hybridized with the [32P]-labeled tRF3E-MRC probe at 56°C essentially as described by Sambrook and Russell (53). Lanes M1-M3, normal mammary glands; T1-T3, mammary tumors; H, heart; B, brain; K, kidney; L, liver; S, spleen. As standards, known quantities of a synthetic tRF3E were loaded (3 ng in E and 2.5 ng in F ) and used to determine the absolute values for tRF3E (0.95–1.95 ng) and tRNAGlu(;41 ng)
contained in 10 mg of total RNA. C1 and C2 represent the amplification products of the full-length tRNAGlu and tRF3E,
respectively, as described in Fig. 1. The slight difference in migration between the native tRF3E and the synthetic one can be due to the modified bases that are present only in the endogenous tRF3E and by the run under a weaker denaturation condition in agarose gels. These differences were lost in PAGE-urea (6 M) gels (Fig. 1B and unpublished results).
of total RNA per cell. Because no difference in the ex-pression of tRNAGlubetween normal and tumoral tissues is observed, we suppose that tRF3E production is con-trolled at the level of tRNA cleavage and its processing is dysregulated in BC. Moreover, northern blots show that tRF3E is expressed in mammary glands but not in other organs (heart, brain, kidney, liver, and spleen) explanted from WT FVB mice (Fig. 2F).
Next, we extended these analyses by examining the tRF3E level in human plasma from 23 healthy individuals and 47 patients with HER2-positive BC by qRT-PCR. As shown in Fig. 3A, plasma belonging to healthy volunteers contains;3-fold higher values of tRF3E than those found in BC samples. These data are consistent with those obtained in mice and provide further evidence that breast carcinogenesis is associated with an altered expression of circulating tRF3E. The inverse correlation between tRF3E levels and human tumor progression was further dem-onstrated by evaluating the tRF3E levels in the plasma of 3 groups of patients with HER2-positive BC, representing different stages of cancer progression: 21 patients receiving adjuvant chemotherapy, 17 patients receiving neo-adjuvant chemotherapy, and 9 metastatic patients (clinical data are provided in Supplemental Table S1). As shown in Fig. 3B, the concentration of tRF3E significantly decreases in both adjuvant and neoadjuvant groups of patients re-spect to healthy subjects, reaching the lowest values in the case of metastatic patients.
tRF3E specifically interacts with NCL
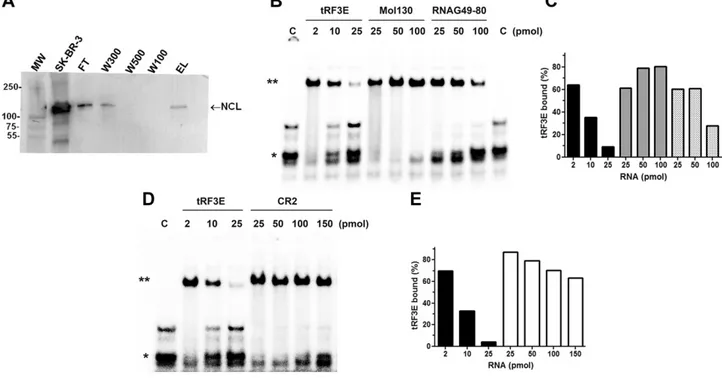
In an attempt to shed light on the pathophysiological role of tRF3E and identify proteins capable of interacting with it, we performed a pulldown assay in which an immobi-lized tRF3E was used to fish out target proteins from an SK-BR-3 crude cellular extract. The pulled-out proteins were analyzed by mass spectrometry (Proteomics Facility, University of Chieti), and only 2 proteins were identified exclusively in the eluate: protein-glutamine g-glutamyl-transferase E and NCL (Supplemental Fig. S2). Between them, we have focused on NCL because it is a known RNA-binding protein that has a role in cancer. The pres-ence of NCL in the pulldown elution sample, analyzed by mass spectrometry, was confirmed by immunoblotting (lane EL, Fig. 4A). Thus, we analyzed in depth the
interaction of tRF3E with NCL by EMSA, and we found that NCL interacts with tRF3E with a high binding affinity. In fact, the dissociation constant (KD) of the NCL-tRF3E
complex is;0.12 mM (Supplemental Fig. S3). This prom-ising result prompted us to further investigate the tRF3E-NCL interaction by a competitive EMSA in which NCL-tRF3E complexes, upon formation, were subjected to competition by a large excess of either specific or un-specific RNAs. As shown in Fig. 4B, D, the addition of unspecific RNAs such as Mol130 and CR2, up to 100 and 150 pmol, respectively, does not cause the dissociation of NCL-tRF3E complexes, whereas an effect (reduction by half) is obtained only with the highest amount (100 pmol) of a third unspecific RNA (RNAG49-80). On the contrary, when low concentrations of tRF3E itself are used as com-petitor, the preformed NCL-tRF3E complex suddenly dissociates. In fact, a clear destabilization of NCL-tRF3E interaction is observed by adding 10 pmol of cold tRF3E, and ,10% of complexes are recovered after incubation with 25 pmol of tRF3E. These data demonstrate that tRF3E selectively interacts with NCL displaying a 10-, 25-, and 35-fold higher binding affinity than that obtained with RNAG49-80, Mol130, and CR2, respectively (Fig. 4C, E). Furthermore, the interaction of NCL with tRF3E seems highly cooperative (Supplemental Fig. S4). In fact, at low concentrations of the tRF3E probe (C1), the initial occu-pancy of sites with higher binding affinity, located on RBDs 1 and 2 (RBD1-2, see in silico data), causes a poor yield of NCL-tRF3E complexes. As the total amount of RNA increases due to the addition of Mol130 or tRF3E, the capability of NCL to bind RNA largely rises because of the possible involvement of all 4 RBDs. Notably, the extent of cooperativity drastically differs between the 2 RNAs tested because comparable effects required a 50-fold ex-cess of Mol130 vs. a 5-fold exex-cess of tRF3E (Supplemental Fig. S4).
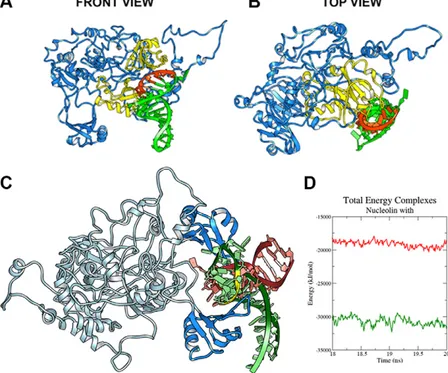
Molecular modeling of NCL-tRF3E complex Because the crystallographic structure of the whole NCL is not available, the in silico complete protein structure was built in blocks starting from the frag-ments previously crystallized (PDB codes 2KRR, 2FG9, 2FG8) corresponding to the RBDs of NCL (Supple-mental Fig. S5). In parallel, 5 optimal models for tRF3E
Figure 3. BC causes a decrease of circulating tRF3E in humans. A) Relative quantification of tRF3E on plasma of healthy volunteers (n = 23) and patients with BC (n = 47) was carried out by qRT-PCR. Statistical analysis was calculated with a Student’s t test. B) tRF3E level was measured in human patients belonging to one of the following 4 classes: healthy people (n = 23), patients receiving adjuvant chemotherapy (n = 21), patients receiving neoadjuvant chemo-therapy (n = 17), and metastatic patients (n = 9). Values are means6SEM. Statistical analysis was
calculated with ANOVA plus Newman-Keuls multiple comparison test. ***P, 0.001 (signif-icance when compared with healthy samples).
structure were obtained (unpublished results) using SimRNAweb server (31). Then, the first 2 tRF3E docked structures (namely poses 1 and 2), showing much lower energy than the overall models, have been chosen to perform an NCL-tRF3E docking prediction and to study in depth the dynamic of this physical RNA-protein interaction. Docking studies reveal that tRF3E is located between 2 RBDs, and the identified binding site involves mostly RBD1 and RBD2 and the loop between them. In fact, the structure shown in Fig. 5A, B indicates that NCL (in blue) binds tRF3E (in green) via a preferential interaction between RBD1-2 (both in yellow) and the known consensus sequence UCCCG (in red), located in position 19–23 on tRF3E. The best en-ergy model presents 22 NCL residues interacting with 9 tRF3E nucleotides, for a total of 265 interactions. Im-portantly, 4 out of 5 nt that comprise the NCL consensus motif are involved in this interaction (unpublished re-sults). In light of these promising results, we compared the binding affinity of NCL for tRF3E with that for preribosomal rRNA (pre-rRNA), a well-known target of NCL (32). To this goal, the free energy of binding of NCL-tRF3E and NCL-pre-rRNA complexes, recon-structed from crystallographic structure (1rkj PDB code), has been evaluated using the MM-PBSA, method and the structures of these 2 NCL-RNA complexes were
superimposed (Fig. 5C). tRF3E has a considerable major binding affinity with respect to 1rkj-RNA, as evidenced by greater negative values of DGcomplex(230736.43 kJ/
mol for NCL-tRF3E vs.219140.93kJ/mol for NCL-1rkj-RNA) (Fig. 5D). This computer analysis has been used to further investigate the role of the 4 RBDs in the for-mation of NCL-tRF3E complexes. Data reported in Supplemental Fig. S6 indicate that both the 2 lowest energy poses of tRF3E (poses 1 and 2) bind to RBD1-2 more stably (lower energies) than 1rkj–pre-rRNA. However, the binding affinity of tRF3E significantly decreases when bound to the less favorite site RBD3-4, reaching values similar to those of pre-rRNA. These outcomes remark the very strong interaction of tRF3E with RBD1-2 of NCL and are in accordance with the very small distance (,2 ˚A) measured between these binding domains and the sequence UCCCG on tRF3E (unpublished results). Of note, we found a drastic de-crease of free energy values when RBD1-2 and RBD3-4 were simultaneously occupied by 2 molecules of tRF3E (Supplemental Fig. S6). The binding affinity for this model (279128.70 kJ/mol) is less than half of that esti-mated for the system containing only 1 tRF3E molecule bound to RBD1-2, strongly confirming the cooperative effect observed in EMSA experiments (Supplemental Fig. S4).
Figure 4. NCL selectively binds tRF3E. A) Samples taken at different steps of pulldown experiment were analyzed by Western blot using an antibody anti-NCL. After an initial saturation of the immobilized tRF3E that causes the release of the excess of NCL from the resin (FT and W300), the protein disappears in the following washes (W500 and W100) to come again in the eluate (EL). Lane MW, MW marker; lane SK-BR-3, 40 mg of SK-BR-3 crude protein extracts; lane FT,flow through contains the proteins unbound to the immobilized tRF3E; lanes W300, W500, and W100 are the washes at the indicated concentrations (mM) of NaCl; lane EL is the eluted NCL from tRF3E-resin. B–D) EMSA was carried out in the presence of a fixed concentration of purified NCL (0.5 mM) and 0.25 pmol/sample of [32P]-labeled tRF3E (see Materials and Methods), with the exception that, once the NCL-tRF3E complexes were formed, the indicated amounts of cold NCL-tRF3E, Mol130, RNAG49-80 (B), and CR2 (D) RNAs were added. “C” is the control sample in absence of protein, and the electrophoretic migration of free tRF3E is indicated with a single asterisk. Double asterisk indicates RNA-NCL complexes. Signals associated to bound tRF3E were quantified and expressed as percentage of total radioactivity (C, E ). Black, dark gray, light gray, and white bars correspond to tRF3E vs. tRF3E, tRF3E vs. Mol130, tRF3E vs. RNAG49-80, and tRF3E vs. CR2 competitions (%), respectively.
tRF3E causes derepression of p53 expression and inhibits cell proliferation
Given that tRF3E plasma levels inversely correlate with tumor progression, and considering the ability of tRF3E to strongly interact with NCL, we hypothesized that such tRF might act to suppress cancer progression via an NCL-mediated mechanism. Thus, we focused our atten-tion on the expression of target transcripts controlled by NCL and playing a role in cancer. In particular, cytoplas-mic NCL can regulate mRNA translation and enhance transcript stability of several tumor progression genes, including the antiapoptotic factor Akt (15). Confocal analysis of SK-BR-3 cells transfected with biotinylated tRF3E provided direct evidence of the cytoplasmic locali-zation of tRF3E, compatible with its ability to interact with
cytoplasmic NCL and to modulate target mRNAs trans-lation (Supplemental Fig. S7). In this context, we measured the expression levels of Akt on SK-BR-3 cells transfected with this tRF. Western blot analysis reveals that tRF3E does not affect Akt levels as well as its activation by phosphorylation (Supplemental Fig. S8). On the other hand, NCL can also bind the 59-39UTR base-pairing re-gions of p53 mRNA and suppress protein translation (17, 18). Thus, we investigated the expression of the tumor-suppressor protein p53 by Western blot analysis in SK-BR-3 and MCF7 human BC cell lines transiently transfected with tRF3E. Interestingly, the level of p53 in-creases in a statistically significant manner in both SK-BR-3 and MCF7 cells upon tRF3E treatment (Fig. 6). On the contrary, CR2, chosen as control RNA because of its weak interaction with NCL as determined by EMSA (Fig. 4),
Figure 5. In silico study of the NCL-tRF3E interaction. A, B) Front (A) and top (B) views of the NCL-tRF3E complex. The lowest energy structure is shown: tRF3E in green, NCL in blue, with the RBD1-2 in yellow. The sequence UCCCG of tRF3E is highlighted in red. C ) Superimposition of the structures of NCL-tRF3E and NCL–pre-rRNA complexes. NCL is represented as light and deep blue ribbons, 1rkj–pre-rRNA is in red, tRF3E (pose 1) is in green, and the NCL target sequence is high-lighted (yellow). D) The 1rkj PDB structure is available on Protein Data Bank (PDB). MM-PBSA energy was calculated on 20 ns MD simulations and DG values for NCL-tRF3E (green curve), and NCL-1rkj-RNA (red curve) complexes are plotted vs. time.
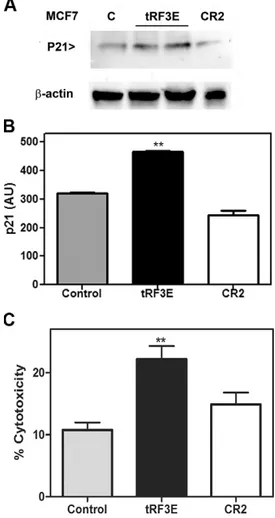
Figure 6. tRF3E induces p53 expression. A, B) Representative image of Western blot analysis for the expression of p53 on SK-BR-3 (A) and MCF7 (B) cells transiently transfected with tRF3E or CR2 RNA or treated with lipofect-amine alone as control (C) for 24 h. Forty micrograms of protein extracts were analyzed for each cell line. C, D) Densitometric quanti-fication of p53 expression normalized on b-actin (loading control), expressed as arbi-trary units (AUs), analyzed from 3 independent experiments. Values are means 6 SEM. The significance was determined by Student’s t test: samples vs. control. *P, 0.05, **P , 0.01.
does not induce any considerably enhancement of p53 synthesis. These results suggest that tRF3E, by competitive binding, is able to displace NCL from p53 mRNA, thereby alleviating the NCL-mediated translational repression. In turn, increased levels of p53 protein, expressed as WT protein in MCF7 cells, may activate p53-responsive target genes inducing growth arrest and cell death (33). Unlike MCF7 cells, SK-BR-3 cells carry endogenously mutant p53His175, which lacks transcriptional activity. Thus, we chose MCF7 cells to evaluate the ability of exogenous tRF3E to induce the synthesis of p21, a key mediator of p53-dependent cell cycle arrest (34), and consequently to modulate cell proliferation. As shown in Fig. 7, MCF7 cells
transfected with tRF3E display an up-regulation of p21 expression coupled with a significantly reduced cell viability.
DISCUSSION
tRFs represent an important new category of regulatory molecules that can be deregulated in human cancers (35). In this study, we report the identification by cloning and sequencing of a new circulating 32 nt tRF (tRF3E) deriving from the 39-end of the mature tRNAGluTTC. So far, very little is known on tRNA fragments biology, and much information about their biogenesis is still lacking. tRF3E shows particular features and hardly fits into the current classification. Indeed, it is slightly longer than canonical tRFs (median length of 22 nt) and shorter than tRNA halves. Although we cannot exclude the involvement of different RNases, analysis of the cleavage site suggests that tRF3E biogenesis could be catalyzed by Dicer, a member of the RNase III family, playing a key role also in RNA in-terference and miRNA pathways. However, differently from other tRFs processed by Dicer at D and T loops (36, 37), tRF3E seems to originate by a noncanonical Dicer cleavage at the anticodon stem of the mature tRNAGlu. This position was detected by an algorithm developed by Ahmed et al. (38) and was experimentally confirmed by the discovery of the circulating 59-tRF (tRF5E), the other fragment derived from tRNAGludigestion.
We provide evidence that tRF3E is constitutively and specifically expressed (up to;5% of tRNAGlu) by mouse mammary glands, but its expression is lost in HER2-positive BC, suggesting a role in the pathogenesis of this disease. Accordingly, we demonstrate that circulating tRF3E is significantly higher in healthy people with respect to patients with BC and progressively decreases during tumor development, reaching the lowest values in the metastatic status. Given that mature tRNAGluexpression is constant between normal and tumor tissues, we hypoth-esized that tRF3E reduction in breast carcinomas was caused by a dysregulation of its processing due to a de-creased Dicer expression, in agreement with the pre-viously reported down-regulation of Dicer in breast, ovarian, and lung cancers and its association with ag-gressive disease and poor overall survival (39–41). How-ever, a northern blot analysis reveals that Dicer mRNA is not transcriptionally down-regulated in BC samples (un-published results), suggesting that a more complex mode of regulation, such as modifications of protein activity, is likely to be responsible for the observed differences in tRF3E production. Alternatively, we can speculate that differences in posttranscriptional modifications such as methylation might protect the precursor tRNA from pro-cessing by Dicer (42). However, further investigation of mechanisms governing tRF3E biogenesis will help to un-derstand the upstream cause of its repression in cancer.
Screens to identify endogenous binding partners of tRF3E have disclosed that this tRF specifically binds NCL with a high affinity. NCL is a multifunctional protein lo-calized in different subcellular compartments (12). Cyto-plasmic NCL has been shown to bind to several cellular
Figure 7. tRF3E affects p21 expression and cell viability. A) Representative image of Western blot analysis for the expression of p21 on MCF7 cells transiently transfected with tRF3E (in duplicate) or CR2 or treated with lipofectamine alone as control (C) for 24 h. Forty micrograms of protein extracts were analyzed for each cell line. B) Densitometric quantification of p21 expression normalized by b-actin (loading control), expressed as arbitrary units (AUs), from 3 independent experiments. Values are means 6 SEM. The
significance was determined by the 1-way ANOVA test followed by Tukey’s multiple comparisons test. **P = 0.001. C) MTT assay of MCF7 cells transiently transfected with tRF3E or CR2 or treated with lipofectamine alone as control for 48 h. Results are expressed as percentage of cell cytotoxicity relative to control cells. Values are means 6 SEM. The significance was
determined by Student’s t test: samples vs. control. **P = 0.0085.
mRNAs, regulating their stability or translation (15, 17, 43, 44). It is known that NCL binds RNAs by recognizing a G-rich signature motif (U/G)CCCG(A/G) within a stem-loop structure (15, 32). tRF3E satisfies these 2 re-quirements, being highly structured and bearing the NCL consensus sequence, at position 19–24,that, according to in silico data, is preferentially recognized by RBD1-2 of NCL. The high selectivity of NCL-tRF3E interaction is demon-strated by the following: 1) the low dissociation con-stant (KD ;120 nM) comparable to that estimated by
Ghisolfi-Nieto et al. (32) for pre-rRNA, a well-known target of NCL; 2) the clear difference in the kinetic of dissociation among tRF3E and control RNAs in competitive EMSA; and 3) the binding energy for NCL-tRF3E complex, cal-culated in silico, that is even lower than that for NCL– pre-rRNA complex. Moreover, we provide experimental evidence that NCL cooperatively binds tRF3E. This result is strengthened by computer prediction revealing that, in addition to RBD1-2, also RBD3-4 can participate in the binding of tRF3E. Of note, the simultaneous occupancy of all RBDs determines a drastic stabilization of the NCL-tRF3E complex. This is not a secondary aspect be-cause a cooperative bond would lower the amount of tRF3E required to saturate all NCL binding sites, thus af-fecting the regulatory function of the protein. Mechanis-tically, tRF3E, thanks to its high cellular concentration and binding properties, is potentially able to displace specific mRNAs controlled by NCL. In particular, because NCL can suppress p53 translation by interacting with a double-stranded RNA region formed by an intra-molecular base pairing of p53 mRNA (18), we propose a model in which tRF3E, by competing for NCL, causes the release of p53 mRNA promoting its translation. p53 is a well-known tumor suppressor that may induce growth arrest or cell death in response to diverse stress signals (33). Increased translation of p53 mRNA has been de-fined as a critical step in the induction of the p53 protein (17). The suggested mechanism of action of tRF3E is supported by experimental evidence obtained in SK-BR-3 and MCF7 BC cells transfected with tRFSK-BR-3E. In fact, the level of p53 significantly increases in both SK-BR-3 and MCF7 cells upon tRF3E treatment. In our system, the enhancement of the p53 protein level is concomitant with an induction of p21 and a reduced proliferation rate in MCF7 cells, which harbor the p53 WT gene, suggesting that loss of tRF3E expression may confer a growth advantage to malignant cells.
In the last few years, others have reported a down-regulation of specific tRFs in cancer and proposed a tumor-suppressive role for these molecules. Balatti et al. (45) demonstrated that the ts-4521/3676 cluster, ts-46, and ts-47 were lower expressed in chronic lymphocytic leu-kemia and lung cancer, whereas Maute et al. (46) observed that tRF CU1276, able to modulate DNA damage response pathways, was strongly down-regulated in lymphomas. Another tRF, called tRF/miR-1280, has been described to suppress colorectal cancer growth and metastasis by repressing Notch signaling pathways (47). Although some studies suggest that tRFs function like miRNAs (46), recent evidence has demonstrated that tRFs can also operate through completely different mechanisms, e.g., tRFs can
control retrotransposition (48), regulate ribosome bio-genesis (49), and modulate translation by displacing eukaryotic initiation factors from mRNAs (50) or by competing with mRNA binding to the ribosome (51).
Here, we unveil a tumor-suppressive activity for tRF3E that is mediated by a physical interaction with the RNA-binding protein NCL. Similarly, a protein-dependent dis-placement mechanism was demonstrated in a previous study for specific tRFs, derived from tRNAGlu, tRNAAsp, tRNAGly, and tRNATyr(52). In that case, the researchers found that tRFGlubinds YBX1, an RNA-binding protein that, besides controlling many key cellular processes, is able also to stabilize oncogenic transcripts enhancing their translation. Furthermore, this tRFGluwas significantly in-duced by hypoxia in nonmetastatic BC cells, but its ex-pression was reduced in highly metastatic cells. Although tRFGluidentified by Goodarzi et al. (52) and tRF3E derive from the same mature tRNA, they share only a 7-nt stretch (from position 41 to position 47) and exhibit distinctive functions that correlate with their different protein target. Reported data reinforce the idea that tRFs are versatile and powerful tools to regulate gene expression and indicate that tRF3E acts as an endogenous inhibitor of NCL dis-playing anticancer activities. Although we focused on the enhancement of p53 expression by tRF3E, further in-vestigation of additional tRF3E targets will help to fully clarify its biologic function.
ACKNOWLEDGMENTS
The authors thank Luca Digiacomo (Univeristy of Rome, La Sapienza, Italy) for valuable support in confocal imaging acquisition, and Stefania Angelucci (University of Chieti) and Fabrizio Di Giuseppe (Proteomics Facility Unit, University of Chieti, Chieti, Italy) for mass spectrometry analysis. This work was supported by a Fondi Ricerca Ateneo grant from Camerino University (BVI000002 to M.F.), and was partially supported by the Italian Association of Cancer Research (AIRC; IG 11889 to A.A.). The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
M. Falconi, M. Giangrossi, M. Elexpuru Zabaleta, J. Wang, V. Gambini, M. Tilio, D. Bencardino, C. Marchini, and A. Amici performed experiments; S. Occhipinti and B. Belletti contributed new reagents and human blood samples; E. Laudadio and R. Galeazzi designed and performed in silico studies; M. Falconi, M. Giangrossi, C. Marchini, and A. Amici designed research and analyzed data; M. Falconi and C. Marchini wrote the paper; and all authors reviewed the results and approved the final version of the manuscript.
REFERENCES
1. Sorlie, T., Tibshirani, R., Parker, J., Hastie, T., Marron, J. S., Nobel, A., Deng, S., Johnsen, H., Pesich, R., Geisler, S., Demeter, J., Perou, C. M., Lønning, P. E., Brown, P. O., Børresen-Dale, A. L., and Botstein, D. (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 100, 8418–8423
2. Calin, G. A. (2019) The noncoding RNA revolution-three decades and still going strong! Mol. Oncol. 13, 3
3. Yuan, Y., and Weidhaas, J. B. (2019) Functional microRNA binding site variants. Mol. Oncol. 13, 4–8
4. Raina, M., and Ibba, M. (2014) tRNAs as regulators of biological processes. Front. Genet. 5, 171
5. Anderson, P., and Ivanov, P. (2014) tRNA fragments in human health and disease. FEBS Lett. 588, 4297–4304
6. Romano, G., Veneziano, D., Acunzo, M., and Croce, C. M. (2017) Small non-coding RNA and cancer. Carcinogenesis 38, 485–491 7. Li, S., Xu, Z., and Sheng, J. (2018) tRNA-derived small RNA: a novel
regulatory small non-coding RNA. Genes (Basel) 9, E246
8. Kuscu, C., Kumar, P., Kiran, M., Su, Z., Malik, A., and Dutta, A. (2018) tRNA fragments (tRFs) guide Ago to regulate gene expression post-transcriptionally in a Dicer-independent manner. RNA 24, 1093–1105
9. Vojtech, L., Woo, S., Hughes, S., Levy, C., Ballweber, L., Sauteraud, R. P., Strobl, J., Westerberg, K., Gottardo, R., Tewari, M., and Hladik, F. (2014) Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 42, 7290–7304
10. Huang, X., Yuan, T., Tschannen, M., Sun, Z., Jacob, H., Du, M., Liang, M., Dittmar, R. L., Liu, Y., Liang, M., Kohli, M., Thibodeau, S. N., Boardman, L., and Wang, L. (2013) Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics 14, 319
11. Mongelard, F., and Bouvet, P. (2007) Nucleolin: a multiFACeTed protein. Trends Cell Biol. 17, 80–86
12. Jia, W., Yao, Z., Zhao, J., Guan, Q., and Gao, L. (2017) New perspectives of physiological and pathological functions of nucleolin (NCL). Life Sci. 186, 1–10
13. Pichiorri, F., Palmieri, D., De Luca, L., Consiglio, J., You, J., Rocci, A., Talabere, T., Piovan, C., Lagana, A., Cascione, L., Guan, J., Gasparini, P., Balatti, V., Nuovo, G., Coppola, V., Hofmeister, C. C., Marcucci, G., Byrd, J. C., Volinia, S., Shapiro, C. L., Freitas, M. A., and Croce, C. M. (2013) In vivo NCL targeting affects breast cancer aggressiveness through miRNA regulation. J. Exp. Med. 210, 951–968; erratum: 214, 1557
14. Berger, C. M., Gaume, X., and Bouvet, P. (2015) The roles of nucleolin subcellular localization in cancer. Biochimie 113, 78–85 15. Abdelmohsen, K., Tominaga, K., Lee, E. K., Srikantan, S., Kang,
M. J., Kim, M. M., Selimyan, R., Martindale, J. L., Yang, X., Carrier, F., Zhan, M., Becker, K. G., and Gorospe, M. (2011) Enhanced translation by nucleolin via G-rich elements in coding and non-coding regions of target mRNAs. Nucleic Acids Res. 39, 8513–8530
16. Zhang, J., Tsaprailis, G., and Bowden, G. T. (2008) Nucleolin stabilizes Bcl-X L messenger RNA in response to UVA irradiation. Cancer Res. 68, 1046–1054
17. Takagi, M., Absalon, M. J., McLure, K. G., and Kastan, M. B. (2005) Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell 123, 49–63
18. Chen, J., Guo, K., and Kastan, M. B. (2012) Interactions of nucleolin and ribosomal protein L26 (RPL26) in translational control of human p53 mRNA. J. Biol. Chem. 287, 16467–16476
19. Andreani, C., Bartolacci, C., Wijnant, K., Crinelli, R., Bianchi, M., Magnani, M., Hysi, A., Iezzi, M., Amici, A., and Marchini, C. (2017) Resveratrol fuels HER2 and ERa-positive breast cancer behaving as proteasome inhibitor. Aging (Albany N.Y.) 9, 508–523
20. Tilio, M., Gambini, V., Wang, J., Garulli, C., Kalogris, C., Andreani, C., Bartolacci, C., Elexpuru Zabaleta, M., Pietrella, L., Hysi, A., Iezzi, M., Belletti, B., Orlando, F., Provinciali, M., Galeazzi, R., Marchini, C., and Amici, A. (2016) Irreversible inhibition of D16HER2 is necessary to suppress D16HER2-positive breast carcinomas resistant to Lapatinib. Cancer Lett. 381, 76–84
21. Kalogris, C., Garulli, C., Pietrella, L., Gambini, V., Pucciarelli, S., Lucci, C., Tilio, M., Zabaleta, M. E., Bartolacci, C., Andreani, C., Giangrossi, M., Iezzi, M., Belletti, B., Marchini, C., and Amici, A. (2014) Sanguinarine suppresses basal-like breast cancer growth through dihydrofolate reductase inhibition. Biochem. Pharmacol. 90, 226–234
22. Yang, J., Yan, R., Roy, A., Xu, D., Poisson, J., and Zhang, Y. (2015) The I-TASSER suite: protein structure and function prediction. Nat. Methods 12, 7–8
23. Fiser, A., and Sali, A. (2003) ModLoop: automated modeling of loops in protein structures. Bioinformatics 19, 2500–2501
24. Shin, W.-H., Lee, G. R., Heo, L., Lee, H., and Seok, C. (2014) Prediction of protein structure and interactions by Galaxy protein modelling programs. Bio Design 2, 1–11
25. Abraham, M. J., Murtola, T., Schulz, R., P´all, S., Smith, J. C., Hess, B., and Lindahl, E. (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to super-computers. SoftwareX 1–2, 19–25
26. Marchini, C., Gabrielli, F., Iezzi, M., Zenobi, S., Montani, M., Pietrella, L., Kalogris, C., Rossini, A., Ciravolo, V., Castagnoli, L., Tagliabue, E., Pupa, S. M., Musiani, P., Monaci, P., Menard, S., and Amici, A. (2011) The human splice variant D16HER2 induces rapid tumor onset in a reporter transgenic mouse. PLoS One 6, e18727
27. Bartolacci, C., Andreani, C., Curcio, C., Occhipinti, S., Massaccesi, L., Giovarelli, M., Galeazzi, R., Iezzi, M., Tilio, M., Gambini, V., Wang, J., Marchini, C., and Amici, A. (2018) Phage-based anti-HER2 vaccina-tion can circumvent immune tolerance against breast cancer. Cancer Immunol. Res. 6, 1486–1498; erratum: 7, 526
28. Segatto, I., Zompit, M. M., Citron, F., D’Andrea, S., Vinciguerra, G. L. R., Perin, T., Berton, S., Mungo, G., Schiappacassi, M., Marchini, C., Amici, A., Vecchione, A., Baldassarre, G., and Belletti, B. (2019) Stathmin is required for normal mouse mammary gland development and D16HER2-driven tumorigenesis. Cancer Res. 79, 397–409
29. Roberts, T. C., Coenen-Stass, A. M., and Wood, M. J. (2014) Assessment of RT-qPCR normalization strategies for accurate quan-tification of extracellular microRNAs in murine serum. PLoS One 9, e89237
30. Li, Y., and Kowdley, K. V. (2012) Method for microRNA isolation from clinical serum samples. Anal. Biochem. 431, 69–75
31. Magnus, M., Boniecki, M. J., Dawson, W., and Bujnicki, J. M. (2016) SimRNAweb: a web server for RNA 3D structure modeling with optional restraints. Nucleic Acids Res. 44, W315–W319
32. Ghisolfi-Nieto, L., Joseph, G., Puvion-Dutilleul, F., Amalric, F., and Bouvet, P. (1996) Nucleolin is a sequence-specific RNA-binding protein: characterization of targets on pre-ribosomal RNA. J. Mol. Biol. 260, 34–53
33. Lacroix, M., Toillon, R. A., and Leclercq, G. (2006) p53 and breast cancer, an update. Endocr. Relat. Cancer 13, 293–325
34. He, G., Siddik, Z. H., Huang, Z., Wang, R., Koomen, J., Kobayashi, R., Khokhar, A. R., and Kuang, J. (2005) Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene 24, 2929–2943
35. Sun, C., Fu, Z., Wang, S., Li, J., Li, Y., Zhang, Y., Yang, F., Chu, J., Wu, H., Huang, X., Li, W., and Yin, Y. (2018) Roles of tRNA-derived fragments in human cancers. Cancer Lett. 414, 16–25
36. Kurzynska-Kokorniak, A., Koralewska, N., Pokornowska, M., Urbanowicz, A., Tworak, A., Mickiewicz, A., and Figlerowicz, M. (2015) The many faces of Dicer: the complexity of the mechanisms regulating Dicer gene expression and enzyme activities. Nucleic Acids Res. 43, 4365–4380
37. Cole, C., Sobala, A., Lu, C., Thatcher, S. R., Bowman, A., Brown, J. W., Green, P. J., Barton, G. J., and Hutvagner, G. (2009) Filtering of deep sequencing data reveals the existence of abundant Dicer-dependent small RNAs derived from tRNAs. RNA 15, 2147–2160
38. Ahmed, F., Kaundal, R., and Raghava, G. P. S. (2013) PHDcleav: a SVM based method for predicting human Dicer cleavage sites using sequence and secondary structure of miRNA precursors. BMC Bioinformatics14, S9
39. Grelier, G., Voirin, N., Ay, A. S., Cox, D. G., Chabaud, S., Treilleux, I., L´eon-Goddard, S., Rimokh, R., Mikaelian, I., Venoux, C., Puisieux, A., Lasset, C., and Moyret-Lalle, C. (2009) Prognostic value of Dicer expression in human breast cancers and association with the mesenchymal phenotype. Br. J. Cancer 101, 673–683
40. Karube, Y., Tanaka, H., Osada, H., Tomida, S., Tatematsu, Y., Yanagisawa, K., Yatabe, Y., Takamizawa, J., Miyoshi, S., Mitsudomi, T., and Takahashi, T. (2005) Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 96, 111–115 41. Merritt, W. M., Lin, Y. G., Han, L. Y., Kamat, A. A., Spannuth, W. A.,
Schmandt, R., Urbauer, D., Pennacchio, L. A., Cheng, J. F., Nick, A. M., Deavers, M. T., Mourad-Zeidan, A., Wang, H., Mueller, P., Lenburg, M. E., Gray, J. W., Mok, S., Birrer, M. J., Lopez-Berestein, G., Coleman, R. L., Bar-Eli, M., and Sood, A. K. (2008) Dicer, Drosha, and outcomes in patients with ovarian cancer. N. Engl. J. Med. 359, 2641–2650
42. Schaefer, M., Pollex, T., Hanna, K., Tuorto, F., Meusburger, M., Helm, M., and Lyko, F. (2010) RNA methylation by Dnmt2
protects transfer RNAs against stress-induced cleavage. Genes Dev. 24, 1590–1595
43. Ishimaru, D., Zuraw, L., Ramalingam, S., Sengupta, T. K., Bandyopadhyay, S., Reuben, A., Fernandes, D. J., and Spicer, E. K. (2010) Mechanism of regulation of bcl-2 mRNA by nucleolin and A+U-rich element-binding factor 1 (AUF1). J. Biol. Chem. 285, 27182–27191
44. Otake, Y., Soundararajan, S., Sengupta, T. K., Kio, E. A., Smith, J. C., Pineda-Roman, M., Stuart, R. K., Spicer, E. K., and Fernandes, D. J. (2007) Overexpression of nucleolin in chronic lymphocytic leukemia cells induces stabilization of bcl2 mRNA. Blood 109, 3069–3075
45. Balatti, V., Nigita, G., Veneziano, D., Drusco, A., Stein, G. S., Messier, T. L., Farina, N. H., Lian, J. B., Tomasello, L., Liu, C. G., Palamarchuk, A., Hart, J. R., Bell, C., Carosi, M., Pescarmona, E., Perracchio, L., Diodoro, M., Russo, A., Antenucci, A., Visca, P., Ciardi, A., Harris, C. C., Vogt, P. K., Pekarsky, Y., and Croce, C. M. (2017) tsRNA signatures in cancer. Proc. Natl. Acad. Sci. USA 114, 8071–8076
46. Maute, R. L., Schneider, C., Sumazin, P., Holmes, A., Califano, A., Basso, K., and Dalla-Favera, R. (2013) tRNA-derived microRNA modulates proliferation and the DNA damage response and is down-regulated in B cell lymphoma. Proc. Natl. Acad. Sci. USA 110, 1404–1409
47. Huang, B., Yang, H., Cheng, X., Wang, D., Fu, S., Shen, W., Zhang, Q., Zhang, L., Xue, Z., Li, Y., Da, Y., Yang, Q., Li, Z., Liu, L., Qiao, L., Kong, Y., Yao, Z., Zhao, P., Li, M., and Zhang, R. (2017) tRF/miR-1280
suppresses stem cell-like cells and metastasis in colorectal cancer. Cancer Res. 77, 3194–3206
48. Schorn, A. J., Gutbrod, M. J., LeBlanc, C., and Martienssen, R. (2017) LTR-retrotransposon control by tRNA-derived small RNAs. Cell 170, 61–71.e11
49. Kim, H. K., Fuchs, G., Wang, S., Wei, W., Zhang, Y., Park, H., Roy-Chaudhuri, B., Li, P., Xu, J., Chu, K., Zhang, F., Chua, M. S., So, S., Zhang, Q. C., Sarnow, P., and Kay, M. A. (2017) A transfer-RNA-derived small RNA regulates ribosome biogenesis. Nature 552, 57–62
50. Ivanov, P., Emara, M. M., Villen, J., Gygi, S. P., and Anderson, P. (2011) Angiogenin-induced tRNA fragments inhibit translation initiation. Mol. Cell 43, 613–623
51. Gebetsberger, J., Wyss, L., Mleczko, A. M., Reuther, J., and Polacek, N. (2017) A tRNA-derived fragment competes with mRNA for ribosome binding and regulates translation during stress. RNA Biol. 14, 1364–1373
52. Goodarzi, H., Liu, X., Nguyen, H. C., Zhang, S., Fish, L., and Tavazoie, S. F. (2015) Endogenous tRNA-derived fragments sup-press breast cancer progression via YBX1 displacement. Cell 161, 790–802
53. Sambrook, J., and Russell, D. W. (2001) Molecular Cloning. A Laboratory Manual, CSHL Press, Cold Spring Harbor, NY, USA
Received for publication February 14, 2019. Accepted for publication August 19, 2019.