Robotic Liver Surgery: Early Experience From a Single
Surgical Center
Yolanda Quijano, MD, PhD, Emilio Vicente, MD, PhD, FACS, Benedetto Ielpo, MD,
Hipolito Duran, MD, PhD, Eduardo Diaz, MD, Isabel Fabra, MD,
Sergio Olivares, MD, Valentina Ferri, MD, Irene Ortega, MD, Luis Malave´, MD,
Antonio Ferronetti, MD, Giuseppe Piccinni, MD, PhD, and Riccardo Caruso, MD
Introduction: The use of robotic surgery in liver resection is still limited. Our aim is to present our early experience of robotic liver resection.
Materials and Methods: It is a retrospective review of Sanchinarro University hospital experience of robotic liver resection performed from 2011 to 2014. Clinicopathologic characteristics, and peri-operative and postperi-operative outcomes were recorded and analyzed. Results: Twenty-one procedures have been performed and 13 (65%) of them were for malignancy. There were 2 left hepatec-tomies, 1 right hepatectomy, 1 associated liver partition and portal vein ligation staged procedure (both steps by robotic approach), 1 bisegmentectomy and 3 segmentectomies, 9 wedge resections, and 3 pericystectomies. The mean operating time was 282 minutes (range, 90 to 540 min). Overall conversion rate and postoperative compli-cation rate were 4.7% and 19%, respectively. The mean length of hospital stay was 13.4 days (range, 4 to 64 d).
Conclusion: From our early experience, robotic liver surgery is a safe and feasible procedure, especially for major hepatectomies. Key Words: robotic, minimally invasive surgery, hepatectomy
(Surg Laparosc Endosc Percutan Tech 2016;26:66–71)
I
n the last 2 decades, there has been an increased interest in the minimally invasive surgical (MIS) techniques that is still predominantly represented by laparoscopy. Hep-atobiliary resection is among the most complex and chal-lenging of abdominal operations. In this field, laparoscopy has important limitations, especially for major hepatec-tomies, performed in only a few centers. Robotic surgical technologies have been introduced with the goal of improving current outcomes from laparoscopic surgery, enhancing a surgeon’s dexterity in the surgical field through a magnified 3-dimensional view, instruments with 7 degrees of freedom, intuitive hand-control movements, and short-ening the learning curve in MIS techniques.1Although only a few studies have been published describing the technical feasibility and safety of robotic liver resections, an increasing number of major hepatec-tomies have been performed.2Furthermore, in the scientific
literature there are no reports outlining the early practice of robotic liver resection.
The aim of this report is to present the initial experi-ence we have had in our surgical center of robotic liver resection.
MATERIALS AND METHODS
Our center is a private university hospital with an integrated comprehensive oncological center. The robotic program started in October 2010, and up to now, 210 procedures have been performed, including colorectal, hepatopancreatobiliary, gastroesophageal, and retro-peritoneal procedures.
Since the first robotic liver resection was performed in 2011, a database has been developed and data were pro-spectively recorded up to December 2014.
The institutional review board at Madrid Sanchinarro University Hospital approved this study. All operations were performed using a Da Vinci Robotic Surgical System model Si (Intuitive Surgical, Sunnyvale, CA).
A consensus for surgical treatment was reached during a multidisciplinary conference with surgeons, oncologists, gastroenterologists, and radiologists.
Preoperative Study
All patients underwent a preoperative work up including triphase thoracicabdominal computed tomog-raphy (CT) scan, PET-CT scan, magnetic resonance imaging (MRI), or cholangio-MRI when required.
Data Collected
Patient demographics, along with preoperative, peri-operative, and postoperative clinical data were collected from our prospective liver surgery database.
Histologic information, the type of hepatic liver resection, mean operative time, combined procedures, intraoperative bleeding, need for blood transfusion, infor-mation on conversion either to open, laparoscopic, or hand port procedure, and margin status were also collected.
The Surgical Team
Six surgeons, experts in hepatobiliary surgery, per-formed all the procedures under the supervision of the 2 chiefs of surgery. All had extensive advanced laparoscopic experience and had completed a robotic training program.
Surgical Procedure
We standardized positioning and the insertion of the trocars for the majority of patients. Modifications were allowed to suit individual cases.
Received for publication March 16, 2015; accepted November 9, 2015. From the General Surgery Department, Madrid Norte Sanchinarro
San Pablo University Hospital, Madrid, Spain. The authors declare no conflicts of interest.
Reprints: Benedetto Ielpo, MD, General Surgery Department, Madrid Norte Sanchinarro San Pablo University Hospital, Calle On˜a 10, Madrid 28050, Spain (e-mail: [email protected]).
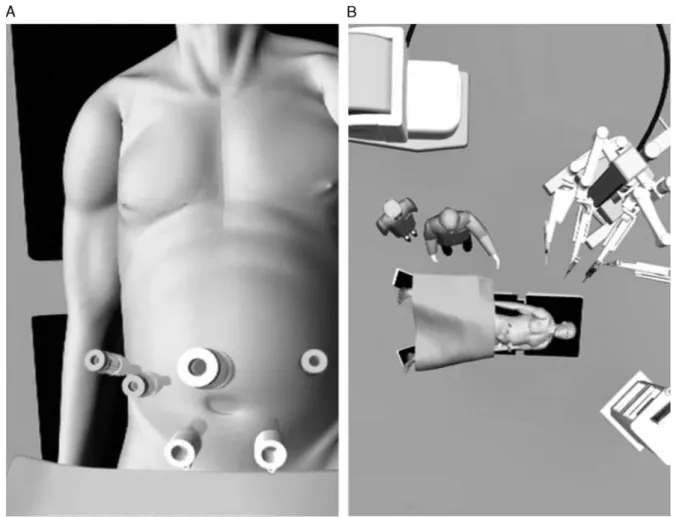
The patient was placed in a 20-degree reverse-Tren-delenburg position with legs apart. The patient was tilted to the left or to the right according to the site of hemi-hepatectomy. After achieving the pneumoperitoneum with a Veress needle, a 12-mm trocar was introduced below the umbilicus, and the other trocars were positioned along a bowl-shaped line passing through the umbilicus completing the docking time, as shown in Figures 1A and B.
The assisting surgeon was positioned between the patient’s legs to perform complementary maneuvers (ie, suction, stapling, retraction, and laparoscopic ultra-sonography) and the scrub nurse and instruments were positioned laterally to the left leg. Usually, for major hep-atectomies 2 laparoscopic-assisted trocars are used.
A 30-degree optic was then introduced through the umbilicus trocar. Following preliminary laparoscopic exploration of the abdominal cavity, intraoperative ultra-sound (Hitachi) was performed to confirm the tumor location and its position in relation to the main vessels. Consequently, we proceeded to liver mobilization, with division of peritoneal attachments and triangular ligaments or falciform ligaments as necessary. For posterior segment and right hepatectomies, the liver was completely freed from the vena cava. The inferior hepatic vena cava was carefully dissected to prevent injury to the right renal and adrenal veins, followed by further piggy-back mobilization of the liver with division between clips of the short hepatic
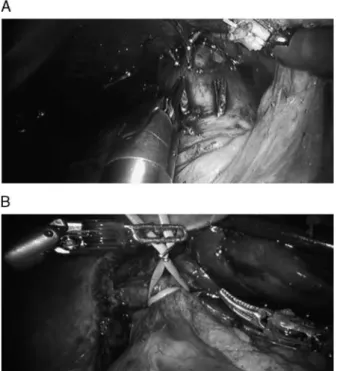
veins that run from the anterior surface of the vena cava directly into the posterior aspect of the liver (Fig. 2A). To gain access to the short hepatic veins, the liver had to be gently lifted and rotated to the left with the robotic arm.
The next step involved entry into the lesser sac through the division of the gastrohepatic ligament. Cholecystectomy and subsequent dissection of the hepatic pedicle were per-formed, passing a vascular sling around the hep-atoduodenal ligament to facilitate the Pringle maneuver, when necessary (Fig. 2B).
For major hepatectomies, hilar dissection included identification and ligation of the right or left hepatic artery, respectively, right or left biliary duct, and careful dissection of the portal vein from its bifurcation into the left and right branches, which are respectively ligated with Hem-o-Lok (Fig. 3A).
The transaction plane was outlined on the Glisson capsule with monopolar diathermy and the third robotic arm was employed to retract and open up the dissection plane. Before liver transection 2-stay sutures were applied at the transaction margins for better retraction (Fig. 3B). The transection was performed in a caudal to cephalic direction, using staplers, a harmonic scalpel, or electro-cautery, according to the local conditions and the surgeons’ preferences.
Small vessels were cauterized, whereas larger vessels and the bile ducts were clipped or sutured. The portal
pedicles and the hepatic vein trunks were dissected and divided using endovascular staplers.
Finally, the specimen was recovered inside a plastic bag through a Pfannenstiel incision.
Hemostasis and biliary fistula were checked. Biliary leaks were sutured with monofilament sutures and several Tachosil patches were placed over the liver transection surface to prevent postoperative bleeding complications.
Intraoperative immunofluorescence was used in major hepatectomies to better define biliary tract location, to better define tumor borders and its precise location and finally to check biliary fistula.
All patients had a drain placed close to the hepatic liver transection surface.
Postoperative Follow-up
Complications were recorded within 60 days of the initial primary procedure. They were classified according to the Dindo-Clavien classification system.3
Posthepatectomy liver failure and bile leakage were reported using consensus definitions and grading systems established by the International Study Group of Liver Surgery.4
Patients were seen in clinic 2 weeks after hospital discharge and then once monthly for the following 3 months unless complications requiring additional visits occurred.
RESULTS
Patient Demographics and Surgical Indications
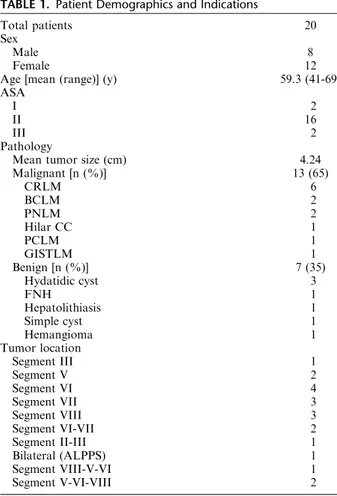
From June 2011 to October 2014, a total of 20 patients underwent 21 robotic liver resections in our surgical unit. These resections were carried out on 8 male and 12 female patients, with a mean age of 59.3 years. The patient’s demographic data are shown in Table 1. Among these procedures, 13 (65%) of them were performed for malig-nant conditions.
Indications for surgery are summarized in Table 1.
Intraoperative Outcomes
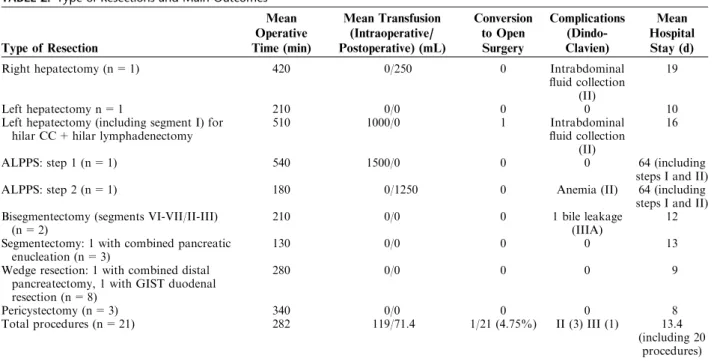
A total of 1 right hepatectomy, 1 left hepatectomy, 1 left hepatectomy extended to segment I (with hilar lym-phadenectomy and with right side biliary resection and hepatojejunal anastomosis for cholangiocarcinoma), 1 associated liver partition and portal vein ligation staged (ALPPS) hepatectomy (I and II steps), 2 bisegmentectomy, 3 segmentectomies, 8 wedge resections, and 3 pericystec-tomies have been performed (Table 2).
Three patients (14%) underwent combined procedures such as distal pancreatectomy resection (n = 1), duodenal gastro intestinal stromal tumor resection (n = 1), and pancreatic enucleation (n = 1) (Table 2).
The overall mean operating time was 282 minutes (range, 90 to 540 min). The mean operative time for each procedure is shown in Table 2.
Overall mean intraoperative blood transfusion was 119 mL (range, 0 to 1000 mL) and they occurred only in major hepatectomies (Table 2).
Conversion to open laparotomy occurred in only the left-extended hepatectomy case (4.7% of all cases). At the end of the surgery and after biliary reconstruction, sig-nificant bleeding occurred from the hepatic venous branch. Therefore, a minilaparotomy was performed and the injured hepatic vein was securely ligated.
Postoperative Outcomes
Postoperative outcomes are shown in Table 2. Com-plications occurred in 5 patients (23.8%). According to the Clavien-Dindo system, none of the patients had grade I complications, 3 patients had grade II complications (14.3%), and 1 had grade IIIA complications (4.76%). No reoperations were needed.
FIGURE 2. A, Retrohepatic inferior cava. B, Preparation for Prin-gle maneuver.
There were no deaths up to 60 days after the operation.
The mean postoperative blood transfusion of the 21 procedures was 83 mL (range, 0 to 1250 mL).
The mean hospital and intensive care stays were 13.4 and 3 days, respectively (range, 4 to 64 d and range, 0 to 13 d) (Table 2). Total hospital stay of the 2 ALPSS steps has been considered as a single procedure. Regarding malig-nancy, observed at pathologic examination, all specimen margins showed to be free of disease. The chol-angiocarcinoma specimen showed a total of 13 resected lymph nodes (T2N0).
The malignant tumor size ranged from 1 to 7 cm with a mean size of 4.3 cm.
DISCUSSION
The advantages of the MIS approach have been well documented, offering better short-term results including shorter hospital stay, reduced postoperative pain, and lower complication rates.5
However, the MIS approach for hepatobiliary disease has grown slowly compared with traditional indications such as for colorectal surgery. There are several obstacles for its widespread use in the hepatobiliary field. Firstly,
liver resection requires a high level of experience and only a few hepatobiliary surgeons have enough MIS skills to deal with it. Secondly, the MIS liver approach is currently mainly represented by laparoscopy, which has its own limitations when performing certain types of liver resection, such as major hepatectomy and posterior segmentectomy. This is mainly because of the reduced freedom of move-ment, precision, and ergonomics of the laparoscopic for-ceps and because the surgeon has to deal with 2-dimen-sional view of the operative field. For this reason, the hand-assisted technique has been proposed to provide a safer and more attainable approach to some of the major laparo-scopic resection.6,7
In contrast, the robotic MIS approach may overcome some of these laparoscopic limitations.
Despite the first laparoscopic liver resection being reported 20 years ago, minor resections including segmen-tectomy, subsegmensegmen-tectomy, and wedge resections with a low number of malignancy are still the most common type of surgeries included in the laparoscopic series.5
Otherwise, since the first liver robotic resection reported 10 years ago, in the current literature, there has been an increased report of major hepatectomies as well as malignancy compared with the laparoscopic approach.2,8,9
To confirm this trend, in the present series, the majority of liver resections were performed on patients having malig-nant tumors (n = 13, 65%). It has been claimed that development of competency for robotic surgery has a shorter learning curve for complex liver procedures than laparoscopy.10 This hypothesis can be supported by our
results: surgeons succeeded in performing 5 major robotic hepatectomies (including ALPPS procedure, the first, up to now, being reported in the literature) within only 3 years of the first robotic liver resection and after only 7 minor robotic procedures. Furthermore, we felt we had enough experience to perform the ALPPS procedure only after 2 robotic major liver procedures.
To further validate this concept, before the robotic program began at our center in 2010, we performed a total of 18 laparoscopic liver resections during 3 years, which included no major hepatectomies and 39% of malignancies. According to our own experience, with the help of a robotic system, major anatomic hepatectomies have become more feasible. This is because the individual dis-section and control of the portal and arterial pedicles are greatly facilitated by endowrist instruments, in a similar manner to open abdominal approach.
These maneuvers allowed a better bleeding control with a shorter Pringle time, otherwise hard to achieve only by laparoscopic approach.
In our series, no Pringle maneuvers were needed in any of the major hepatectomies performed (right, left, and ALPPS procedures), even if the liver hilum was encircled by a loop.
Another important limitation of laparoscopy liver resection is the access to posterior segments, considered much easier using robotics endowrist instruments.9,11Three
successful robotic liver resections have been performed in lesions found in segment VII. Furthermore, easier access to the posterior liver surface, allowed, in the case of a major hepatectomy, preventive hepatic vein control before parenchymal transection.
In our series, 7 patients had liver nodules close to a major liver vessel (5 cases: near hepatic vein, 2 cases: con-nected to the portal branch). Using robotic approach, in
TABLE 1. Patient Demographics and Indications
Total patients 20
Sex
Male 8
Female 12
Age [mean (range)] (y) 59.3 (41-69)
ASA
I 2
II 16
III 2
Pathology
Mean tumor size (cm) 4.24
Malignant [n (%)] 13 (65) CRLM 6 BCLM 2 PNLM 2 Hilar CC 1 PCLM 1 GISTLM 1 Benign [n (%)] 7 (35) Hydatidic cyst 3 FNH 1 Hepatolithiasis 1 Simple cyst 1 Hemangioma 1 Tumor location Segment III 1 Segment V 2 Segment VI 4 Segment VII 3 Segment VIII 3 Segment VI-VII 2 Segment II-III 1 Bilateral (ALPPS) 1 Segment VIII-V-VI 1 Segment V-VI-VIII 2
ALPPS indicates associated liver partition and portal vein ligation staged procedure; BCLM, breast cancer liver metastasis; CC, chol-angiocarcinoma; CRLM, colorectal liver metastasis; GISTLM, gastro-intestinal tumor liver metastasis; PCLM, pulmonary cancer liver metastasis; PNLM, pancreatic neuroendocrine liver metastasis.
these cases, we performed the same parenchyma-preserving resections that originally we would have done by open approach, therefore we avoided carrying out major hep-atectomies. These data were consistent with those showed by Casciola et al.12
Successful tumor-free margin resection was achieved in all these cases. These results were consistent with those reported in the literature.9The oncological results of MIS
liver resection have been showed in several case series and comparative studies with the open approach. Comparative studies with open approach did not find differences in free margin rate and survival.9,13
However, almost all these studies mainly included col-orectal liver metastases and some included hepatocellular carcinomas, where lymphadenectomy was not required. In contrast, with cholangiocarcinomas, the standard therapy consisted not only of negative surgical margin resection but also of extrahepatic bile duct resection and lymphadenec-tomy. The robot’s articulating instruments had 7 degrees of freedom and a magnified 3-dimensional view, which made performing bile duct resection and lymphadenectomy pro-cedures much easier than laparoscopy.
One of the major hepatectomies presented in our series was for a cholangiocarcinoma. In this case, a standard lymphadenectomy was performed along the hilum with at least 13 resected lymph nodes.
Similarly, Giulianotti et al14have demonstrated that in
their case study hilar cholangiocarcinoma is safe and fea-sible even when biliary reconstruction and lymphadenec-tomy are required, as was made in our case.
Also, total robotic ALPPS would not have been fea-sible without robotic approach. The dissection and ligation of right portal vein were also a safe procedure; thanks to the fine and precise movements of the robotic instruments. Most of the studies showed that one of the main concerns with robotic surgery is the longer operative time compared
with laparoscopy and open abdominal surgery. The overall mean operating time of our series was 282 minutes, slightly lower to that reported in literature (291.3 min), but higher when compared with laparoscopy (180 min).5,6 However, analyzing our data, it must be considered that the type of malignancies included cholangiocarcinoma requiring a hilar lymphadenectomy and biliary resection and the type of resection included the first ALPPS totally robotic proce-dure. These 2 surgeries have unequivocally increased the overall operative time. Another reason for such a long operative time when compared with laparoscopy is the concomitant complex procedures performed in our series (14%) such as the case of distal pancreatectomy and enu-cleation and the case of gastro intestinal stromal tumor duodenal tumor with the wedge liver resection.
This longer period of time could probably also be explained by the docking that can take up to 30 minutes for the correct positioning of the robotic arms. The learning curve likely contributed to increase in this time.
The latest generation of the Da Vinci system is the Xi, which in the future should facilitate this maneuver, short-ening this time.
In December 2014, we obtained this new Da Vinci system and we will hopefully find out if the operative time decreases. Furthermore, it should be noted that previous studies on liver robot-assisted surgery report mainly early experiences with this technique. Therefore, the operative time may decrease in larger studies.
Unlike the mean intraoperative blood loss (119 mL), our study shows a slightly higher mean postoperative blood loss (71.4 mL) and overall mean hospital stay (13.4 d) compared with the literature. The higher number of major hepatectomies performed, especially the ALPPS procedure, can justify these data again.
Conversion to laparotomy occurred only in 1 patient (4.7%), a slightly lower rate compared with the overall
TABLE 2. Type of Resections and Main Outcomes
Type of Resection Mean Operative Time (min) Mean Transfusion (Intraoperative/ Postoperative) (mL) Conversion to Open Surgery Complications (Dindo-Clavien) Mean Hospital Stay (d)
Right hepatectomy (n = 1) 420 0/250 0 Intrabdominal
fluid collection (II)
19
Left hepatectomy n = 1 210 0/0 0 0 10
Left hepatectomy (including segment I) for hilar CC + hilar lymphadenectomy
510 1000/0 1 Intrabdominal
fluid collection (II)
16
ALPPS: step 1 (n = 1) 540 1500/0 0 0 64 (including
steps I and II)
ALPPS: step 2 (n = 1) 180 0/1250 0 Anemia (II) 64 (including
steps I and II) Bisegmentectomy (segments VI-VII/II-III)
(n = 2)
210 0/0 0 1 bile leakage
(IIIA)
12 Segmentectomy: 1 with combined pancreatic
enucleation (n = 3)
130 0/0 0 0 13
Wedge resection: 1 with combined distal pancreatectomy, 1 with GIST duodenal resection (n = 8)
280 0/0 0 0 9
Pericystectomy (n = 3) 340 0/0 0 0 8
Total procedures (n = 21) 282 119/71.4 1/21 (4.75%) II (3) III (1) 13.4
(including 20 procedures)
conversion rate reported in the literature (7.8%).15 According to a recent review regarding laparoscopic liver resection, the conversion range was found to be between 0% and 26%, therefore, comparable to the robotic approach. Only Ji et al16found a lower conversion rate for
major robotic hepatectomy compared with laparoscopy (0% vs. 10%).
Most of the time, in MIS liver resections, conversion to laparotomy occurs after an uncontrolled bleeding (34%),16 as occurred in our case. When bleeding occurs,
controlling it laparoscopically can be challenging, resulting in an inevitable conversion. Robotic instruments, instead, allowed better bleeding control through an easier and faster suturing vessel. During our experience of a robotic right hepatectomy, accidental injury to the portal vein occurred as the hilar dissection was performed. It was quickly and successfully controlled using precise longitudinal suturing of the vein injury. Had the laparoscopic approach been used, this would have been a likely cause of conversion.
However, our opinion is that only an expert team of hepatobiliary and laparoscopic surgeons can achieve such good results. Robotic surgery has to be considered as a system, allowing the integration of further techniques such as immunofluorescence.17 This approach may potentially
improve the oncological results of liver resection, promote the preservation of liver parenchyma, and enhance the identification of intrahepatic and extrahepatic biliary ducts. Moreover, the robotic system may simplify the use of other MIS procedures, such as the single port. Once these future applications have been studied, we may finally discover the true advantages of robotic surgery in liver resection.
Another important advantage to consider is the ergo-nomic benefits offered to the surgeon using a robot-assisted system. This is particularly useful in large surgeries like major hepatectomies.
This report is limited in terms of analysis of the eco-nomic factors involved. However, this was not the main aim of our study. We are planning to run a study on cost-effectiveness in the near future; we reckon that high cost is still the main concern of using robotics.
Nevertheless, at the beginning of the robotic program, even smaller operation such as resections of anterior liver lesions should be performed to gain experience for more complex operations where we think the cost-effectiveness of robotic surgery could be more achievable.
Even if this study was not specifically carried out to compare different surgical approaches, it still demonstrates the feasibility and efficacy of surgical robotic treatment, even during an early experience, such as the one presented in this case series.
It should be noted that there have been no randomized clinical trials involving laparoscopic and robotic liver resection; such trial is needed to clarify the risks against benefits and to help standardize the approach.
CONCLUSIONS
According to our early experience from a single sur-gical center, robotic liver resection is a safe and feasible procedure from the beginning of its use, even if major
hepatectomies and a number of malignacies are included. We expect improvement over the course of experience.
ACKNOWLEDGMENTS
The authors thank Isabel de Salas, Pablo Ruiz, and Evelin Marshall for their contributions.
REFERENCES
1. JGiulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic extended right hepatectomy with biliary recon-struction. Laparoendosc Adv Surg Tech A. 2010;20:159–163. 2. Ho CM, Wakabayashi G, Nitta H, et al. Systematic review of
robotic liver resection. Surg Endosc. 2013;27:732–739. 3. Dindo D, Demartines N, Clavien PA. Classification of surgical
complications: a new proposal with evaluation in a cohort of 6,336 patients and results of a survey. Ann Surg. 2004;240:205–213.
4. Rahbari NN, Garden OJ, Padbury R, et al. Post hepatectomy haemorrage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB (Oxford). 2011;13:528–535.
5. Alkhalili E, Berber E. Laparoscopic liver resection for malignancy: a review of the literature. World J Gastroenterol. 2014;20:13599–13606.
6. Vigano L, Tayar C, Laurent A, et al. Laparoscopic liver resection: a systematic review. J Hepatobiliary Pancreat Surg. 2009;16:410–421.
7. Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: the Louisville State-ment. Ann Surg. 2008;250:825–830.
8. Berber E, Akyildiz HY, Aucejo F, et al. Robotic versus laparoscopic resection of liver tumours. HPB (Oxford). 2010;12:583–586.
9. Tsung A, Geller DA, Sukato DC, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg. 2014;259:549–555.
10. Heemskerk J, van Gemert WG, de Vries J, et al. Learning curves of robot-assisted laparoscopic surgery compared with conventional laparoscopic surgery: an experimental study evaluating skill acquisition of robot-assisted laparoscopic tasks compared with conventional laparoscopic tasks in inexper-ienced users. Surg Laparosc Endosc Percutan Tech. 2007;17: 171–174.
11. Boggi U, Caniglia F, Amorese G. Laparoscopic robotassisted major hepatectomy. J Hepatobiliary Pancreat Sci. 2009;21: 3–10.
12. Casciola L, Patriti A, Ceccarelli G, et al. Robot-assisted parenchymal-sparing liver surgery including lesions located inthe posterosuperior segments. Surg Endosc. 2011;25: 3815–3824.
13. Spampinato MG, Coratti A, Bianco L, et al. Perioperative outcomes of laparoscopic and robot-assisted majorhepatecto-mies: an Italian multi-institutional comparative study. Surg Endosc. 2014;28:2973–2979.
14. Giulianotti PC, Coratti A, Sbrana F, et al. Robotic liver surgery: resultsfor 70 resections. Surgery. 2011;149:29–39. 15. Buchs NC, Oldani G, Orci LA, et al. Current status of robotic
liver resection: a systematic review. Expert Rev Anticancer Ther. 2014;14:237–246.
16. Ji WB, Wang HG, Zhao ZM, et al. Robotic-assisted laparoscopic anatomic hepatectomy in China: initial experi-ence. Ann Surg. 2011;253:342–348.
17. Marano A, Priora F, Lenti LM, et al. Applicationof fluorescence in robotic general surgery: review of the literature and state of the art. World J Surg. 2013;37:2800–2811.