Expression of the K303R estrogen receptor alfa mulation induces hormonal resistance in breast cancer
Testo completo
Figura

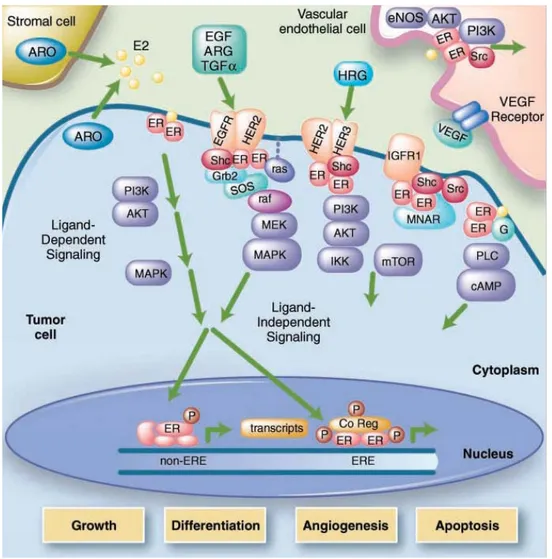


Documenti correlati
Despite these limitations, we have observed that among apparently normal sized fetuses a depressive effect of antenatal steroids is more likely if the weight percentile is in the
Lo scopo di questo sistema è quello di individuare ed identificare strutture ed oggetti sotterranei utilizzando la propagazione di onde elettromagnetiche ad alta
Mario Molteni presenta l’articolo “Organizzazioni non profit e sviluppo: il caso del Cono Norte di Lima”, pubblicato da Clara Caselli nel 2005 nella rivista Non profit, edita da
È giusto altresì tenere a mente che nei libri sul folklore di Cocchiara si valuta Corso come studioso delle tradizioni popolari e non come folklorista di guerra, d’altronde avremo
• For hemispherical domes, the ratio between the buckling loads P cr and F cr (respectively generated by a uniform external pressure and a uniform load over a
Senza un chiaro quadro delle caratteristiche dell’antecedente latino, infatti, risulta diffi- cile stabilire il confine tra l’effettiva attività di compendio del volgarizzatore,
Così come per i musei e tutti gli altri luoghi della cultura, anche le sale da concerto, i teatri o le piazze che portano in scena l’opera lirica o la musica classica
[r]





