https://doi.org/10.1007/s15010-018-1185-6 ORIGINAL PAPER
Endotoxinemia contributes to steatosis, insulin resistance
and atherosclerosis in chronic hepatitis C: the role of pro-inflammatory
cytokines and oxidative stress
Rosa Zampino1 · Aldo Marrone1 · Luca Rinaldi1 · Barbara Guerrera1 · Riccardo Nevola1 · Adriana Boemio1 · Natalina Iuliano1 · Mauro Giordano1 · Nicola Passariello1 · Ferdinando C. Sasso1 · Emanuele Albano2 · Luigi E. Adinolfi1
Received: 7 April 2018 / Accepted: 27 July 2018
© Springer-Verlag GmbH Germany, part of Springer Nature 2018 Abstract
Purpose Endotoxin is a component of the outer membrane of gram-negative bacteria that live in the intestine. Endotoxine-mia is reported in non-alcoholic fatty liver disease and in cirrhotic patients, causing various biological and clinical effects in the host. It is not known whether endotoxinemia occurs in chronic hepatitis C patients (CHC), therefore we evaluated the occurrence of endotoxinemia and its effect on inflammation, liver damage, insulin resistance (IR) and atherosclerosis.
Methods Consecutive CHC patients assessed by liver biopsy were enrolled. Endotoxinemia was evaluated by LAL test. IR was estimated by HOMA-IR. Serum TNF-α, IL-8, adiponectin and MCP-1 were measured with ELISA tests. Oxidative stress was estimated by circulating IgG against malondialdehyde adducts with human serum albumin (MDA-HAS). Carotid atherosclerosis was assessed by ultrasonography.
Results Endotoxinemia was found in 60% of the 126 patients enrolled. A serum level-dependent association between endo-toxinemia, steatosis (p < 0.001) and HOMA-IR (p < 0.006) was observed. Patients with endotoxinemia showed significant increase in TNF-α and IL8 levels. TNF-α correlated with steatosis (p < 0.001) and HOMA-IR (p < 0.03), whereas IL8 cor-related with steatosis (p = <0.001), TNF-α (p < 0.04) and atherosclerosis (p < 0.01). The highest levels of endotoxinemia were associated with oxidative stress and a higher prevalence of carotid atherosclerosis. Multivariate logistic regression analysis showed that the independent factors associated with endotoxinemia were hepatic steatosis, HOMA-IR, IL8 and MDA-HAS.
Conclusions Endotoxinemia occurs with high frequency in CHC patients and contributes to the development of hepatic steatosis, IR and atherosclerosis through increased pro-inflammatory cytokines and oxidative stress. Anti-endotoxin treat-ment could be of clinical relevance.
Keywords Endotoxin · Chronic hepatitis C · Steatosis · Insulin resistance
Introduction
Endotoxin or lipopolysaccharides (LPS) is a component of the outer membrane of gram-negative bacteria and the intestine is a large pool of LPS in the body. A small amount
of LPS can escape from the intestine and through the por-tal vein reach the liver where it is cleared from the reticu-loendothelial system (RES), avoiding a spillover into the systemic circulation. However, endotoxinemia can occur in various clinical conditions such as chronic hepatitis, cirrho-sis, non-alcoholic fatty liver disease (NAFLD) and obesity. Endotoxinemia is known to induce various biological effects in the host involving the liver, cardiovascular, metabolic and neurologic systems [1].
In cirrhotic patients, endotoxinemia may be the result of a decrease in the phagocytic capacity of the liver, a leakage through portosystemic shunts and an increased adsorption of endotoxin due to amplified intestinal permeability [2, 3]. However, irrespective of cirrhosis, endotoxinemia may be a * Luigi E. Adinolfi
1 Department of Medical, Surgical, Neurological, Metabolic, and Aging Sciences, University of Campania “L. Vanvitelli”, Piazza Miraglia, 80138 Naples, Italy
2 Department of Health Sciences and Interdisciplinary Research Center for Autoimmune Diseases, University Amedeo Avogadro of East Piedmont, Novara, Italy
consequence of a high-fat diet intake either through direct diffusion via intestinal paracellular permeability or through absorption by enterocytes during the secretion of chylomi-cron [4], as well as the result of excessive intestinal produc-tion by an altered microbiota.
Human studies have shown that endotoxinemia occurs in both adult and pediatric patients with NALFD [5, 6], pro-moting an increase in the serum TNF-α level that aggra-vates insulin resistance (IR) and liver steatosis [7], and it is claimed that this condition can promote weight gain, the development of NASH, type 2 diabetes mellitus (T2DM) and atherosclerosis [8].
Liver steatosis and IR are features of chronic hepatitis C infection [9] and are of clinical relevance both in the pro-gression of liver injury and in the development of extrahe-patic manifestations [10] and, in particular, T2DM and ath-erosclerosis [11]. Therefore, it is of clinical and therapeutic interest to know whether endotoxinemia is a condition that occurs in chronic hepatitis C and whether it may have a role in the development of HCV-associated conditions such as steatosis, IR, chronic inflammation and atherosclerosis.
Accordingly, the aim of this study was to assess in a series of consecutive patients with liver biopsy proven chronic hepatitis C the prevalence and effects of endotoxinemia and its role in: (a) the progression of hepatic histological dam-age (steatosis, necroinflammatory activity, fibrosis), (b) the development of IR, (c) the production of pro-inflammatory cytokines, oxidative stress and chemokines and, (d) the development of atherosclerosis.
Materials and methods
Patients
Consecutive treatment-naïve chronic hepatitis C patients who underwent liver biopsy for diagnostic purposes were enrolled in this study. Patients with the following conditions were excluded from the study: co-infection with HBV or HIV, alcohol intake greater than 20 g/day, active drug user and with severe comorbidities such as cardiac, pulmonary, renal, rheumatologic, autoimmune and inflammatory gastro-intestinal diseases. None of the patients received steatogenic drugs, antibiotics or probiotics. Patients with urinary tract infection or sepsis as evaluated by urine and blood culture and in general subjects with fever were not included in the study.
At the time of the liver biopsy, a liver ultrasound scan was performed, epidemiological and demographic data were obtained and BMI (kg/m2) was calculated for all patients.
At that time, serum samples were obtained and stored at − 70 °C in pyrogen-free vials and in all patients, hepatic
and renal biochemical tests and blood cells count were determined.
Patients were assessed for carotid intima-media thick-ness (IMT) using high-resolution B-mode ultrasonography (Esaote Techos, Genova, Italy) equipped with a 7.5 MHz linear-array transducer as previously described [10]. IMT > 1 mm was regarded as a cut-off value for carotid ath-erosclerosis (CA). Plaque was defined as a protrusion in the lumen of the vessel of at least 1.5 mm, as measured from the border between the adventitia and median layers.
All patients signed an informed consent and the study protocol in accordance with the ethical guidelines of the 1975 Helsinki Declaration was approved by the Local Eth-ics Committee.
Viral markers
The anti-HCV antibodies were tested by ELISA (Abbott Laboratories, Chicago, IL) and HCV RNA was tested by PCR assay (Amplicor HCV; Roche Diagnostic SpA). HCV RNA serum levels were measured by real-time PCR (Taqman, Applied BioSystems). HCV genotyping was per-formed using a reverse hybridization line probe assay (INNO LiPA HCV assay, 2nd generation, Innogenetics, Zwijndrecht, Belgium) following the manufacturer’s protocol.
Hepatitis B markers and HIV antibodies were assayed using commercially available ELISA tests.
Histology evaluation
Liver histology was evaluated on liver tissue > 1.5 cm through formalin-fixation, followed by paraffin embedment and staining with hematoxylin–eosin, trichrome and Prus-sia blue. “Grading” and “staging” were assessed separately, according to Ishak et al [12]. Different scores were assigned to different levels of steatosis: 0, < 5% of steatosis; 1, 5–10% of hepatocytes with steatosis; 2, 11–30%; 3, 31–60%; 4, > 60% of hepatocytes with steatosis.
Endotoxinemia
Quantification of serum LPS was obtained by Limulus Ame-bocyte Lysate (LAL) with end-point chromogenic method (LAL test, Cape Cod Incorporated/PBI). The cut-off of the test for positivity was calculated using a mean value of 30 healthy controls plus 2 times the standard deviation and was fixed at 0.78 UE/ml.
Insulin resistance
Serum insulin levels were assayed with a radioimmunoassay method (Insulin RIA DSL-1600, Diagnostic System Labo-ratories, USA). The intra- and inter-assay coefficients of
variation ranged from 4.5 to 8.3% and from 4.7 to 12.2%, respectively. The percentages of insulin recovery in analyzed samples ranged from 92 to 119%. In each subject, the degree of insulin resistance was estimated by HOMA-IR using the following formula: [fasting plasma glucose (mmol/L) x fast-ing serum insulin (mIU/L)]/22.5.
Cytokines and chemokines
Serum TNF-α and IL-8 were measured by ELISA test, using the Human ELISA kit (Pierce Biotechnology, Rockford, IL); serum adiponectin levels using the Human Adiponectin ELISA kit (B-Bridge International, Osaka, Japan); MCP-1 was measured using a Human MCP-1 ELISA Kit (Thermo Scientific™ Pierce™).
Measurement of the antibody titers against lipid peroxidation‑derived antigens
Oxidative stress was measured by circulating IgG against malondialdehyde adducts with human serum albumin (MDA-HSA) in HCV-infected patients and in 70 healthy controls. Serum human albumin (HSA) adducts with malon-dialdehyde (MDA) were prepared as previously described [13]. The enzyme-linked immunosorbent assay (ELISA) was performed with patient’s serum as previously reported [13]. The results were expressed by subtracting the back-ground reactivity observed with unmodified HSA. Immu-nological evidence of oxidative stress was considered when the value of anti-MDA-HSA IgG levels exceeded the 95th percentile of the control group (median value, o.d. 490 nm, 0.126 ± 0.052).
Statistical analysis
Values are expressed as the mean ± standard deviation (SD) or median and range. The mean differences between the cases and controls were evaluated by the Student’s t test for parametric data. The Mann–Whitney U test was used to compare non-parametric data between groups. The Chi square test was used to test differences between proportions. Spearman correlation was used to evaluate the association between endotoxin and other variables such as age, sex, BMI, visceral obesity, glycaemia, HOMA-IR, HCV geno-type, serum HCV RNA levels, TNF-α, IL8, adiponectin, MCP-1, hepatic steatosis, HAI, fibrosis and atherosclerosis. The variables significantly associated at univariate analysis were evaluated in the multivariate analysis using a logis-tic regression analysis model. Statislogis-tical analysis was per-formed using the SPSS software for Windows (Version 17.0) (Chicago, IL, USA) and a p value < 0.05 was considered significant.
Results
Patient’s characteristics and prevalence of endotoxinemia
One hundred and twenty-six consecutive chronic hepatitis C patients who underwent liver biopsy were enrolled in this study. Eight patients (6% of the entire eligible population) were not included in the study for one of the aforementioned causes. The median age was 55 years (range 18–68 years), males were 56%, steatosis was present in 54%, cirrhosis in 10% and type 2 diabetes in 10.3% of cases. Carotid ath-erosclerosis was present in 49.2% of patients. Endotox-inemia was found in 76 (60%) patients. Serum anti-MDA-HAS IgG leels, as a marker of oxidative stress-mediated responses, were significantly higher in CHC patients than in healthy controls (o.d 490 nm: 0.228 ± 0.10 vs 0.126 ± 0.052;
p < 0.001, respectively) and the overall prevalence of CHC
patients with immunological evidence of oxidative stress (anti-MDA-HSA IgG levels above the 95th percentile of the control group) was 59%. Table 1 shows the characteristics of patients in relation to the presence or absence of serum endotoxin.
Endotoxinemia, steatosis and IR
Data showed that patients with endotoxinemia had signifi-cantly higher levels of steatosis (p < 0.01; Table 1). Simi-larly, a significant difference between the two groups was present for HOMA-IR (p = 0.05). Such differences were irrespective of type 2 diabetes and/or cirrhosis (data not shown). The correlation test showed a significant associa-tion between LAL and steatosis (r = 0.475; p < 0.001) and HOMA-IR (r = 0.025; p < 0.006).
Considering that the endotoxin effects are reported to be dose-dependent [2], an analysis based on the different serum endotoxin levels was performed. Table 2 shows the analysis of quartile-based data of endotoxinemia and shows a statisti-cally significant difference between the first and fourth quar-tile in steatosis score (p = 0.001), and HOMA-IR (p = 0.001).
Endotoxinemia, inflammatory cytokines and oxidative stress
Endotoxinemia was associated with increased systemic inflammation as indicated by higher levels of TNF-α and IL8, whereas it was not correlated with MCP-1 lev-els (Table 1). Table 2 shows the levels of cytokines in relation to quartile endoxinemia levels. The data showed an increase in serum TNF-α and IL8 levels with the pro-gressive increase in endotoxin levels. Circulating IL8
correlated with steatosis (r = 0.632, p = 0.001), LAL (r = 0.681, p = 0.001) and TNF-α (r = 531, p = 0.042), whereas serum TNF-α levels correlated also with stea-tosis (r = 0.291; p < 0.001) and HOMA-IR (r = 0.129;
p < 0.029). On the contrary, MCP-1 was not associated
with any of the parameters evaluated, regardless of the levels of endotoxinemia. The highest levels of endotox-inemia were also associated with a significant increase in anti-MDA IgG levels (Table 2), indicating that high levels of endotoxinemia are associated with oxidative stress.
Endotoxinemia as a risk factor of atherosclerosis
Chronic HCV infection is known to be associated with ather-osclerosis [11]. In this context, a pro-inflammatory environ-ment and oxidative stress was proposed to play an important role in explaining this association [14, 15]. Therefore, based on the aforementioned data, we investigated the possible role of endotoxinemia in modulating the risk of atheroscle-rosis among HCV patients. The overall prevalence of ath-erosclerosis (increased IMT plus plaque) was no different
Table 1 Characteristics of the patients according to the presence or absence of endotoxinemia
+ IMT increased plus plaque; *Student’s t test ; **Mann–Whitney U test
Variables Endotoxemia
Absent < 0.78 EU/ml Present > 0.78 EU/ml p
No. of patients 50 (40%) 76 (60%)
Age, median (range) 53 (20–68) 55 (18–64) Ns
Male (%) 25 (50%) 46 (60%) Ns
ALT U/L, median (range) 88 (46–461) 93.5 (40–433) Ns HCV RNA, UI/ml × 105 median (range) 4.43 (0.8–56) 7.42 (0.1–32) Ns Cholesterol mg/dl, mean ± SD 173 ± 36 175 ± 39 Ns Triglycerides mg/dl, mean ± SD 115 ± 107 103 ± 40 Ns Glucose mg/dl, median (range) 93 (68–159) 93 (71–201) Ns Insulin μU/ml, median (range) 20.9 (6.5–47.8) 22.9 (6.4–96.4) Ns
HOMA-IR, mean ± SD 4.3 ± 2.6 5.5 ± 2.9 0.05*
Type 2 diabetes 10% 11.4% Ns
TNFα pg/ml, median (range) 12.0 (2.5–25) 51 (6.25–736) 0.016** ADP, U/L median (range) 9.94 (3–12) 8.24 (2.6–26) Ns Serum IL8, (pg/ml), mean ± s.d 10.46 ± 3.7 24.02 ± 19.8 0.001** Serum MCP-1, (pg/ml), mean ± s.d 142.34 ± 39 149.15 ± 42 Ns
MDA, mean ± SD 0.21 ± 0.08 0.24 ± 0.12 Ns
Steatosis score, mean ± s.d 1.2 ± 1.2 2.1 ± 1.4 0.01 HAI score, median (range) 6.0 (1–11) 6.2 (1–13) Ns Fibrosis score, mean ± s.d 2.6 ± 1.1 2.6 ± 1.2 Ns
Cirrhosis 12% 10% Ns
Prevalence of carotid atherosclerosis+ 48% 54.2% Ns
Table 2 Distribution of variables according to the quartiles of endotoxinemia values as evaluated by the LAL test
*Quartile 4 vs quartile 1 + Quartiles 2–4 vs 1
Quartiles 1st 2nd 3rd 4th p
Serum LPS concentration < 0.60 EU/l 0.61–1.20 EU/l 1.21–2.20 EU/l > 2.20 EU/l
Steatosis score mean ± SD 0.8 ± 1.1 1.6 ± 1.5 2.0 ± 1.4 2,4 ± 1.2* 0.001 HOMA-IR mean ± SD 3.9 ± 2.3 4.38 ± 3,3 4.76 ± 1.93 6.37 ± 3.2* 0.005 TNF—pg/ml median (range) 10.9 (2.5–25) 13 (2.6–525) 25.5 (6.2–497) 90.7 (6-736)* 0.03 Serum IL8 (pg/ml) mean ± SD 10.7 ± 3.7 M 26.1 ± 19.1 M 24.3 ± 8.1 29.5 ± 22.6 0.001+ Serum MCP-1 (pg/ml) mean ± SD 130.9 ± 37 M 153.4 ± 59.7 M 144 ± 47.7 157 ± 42.2 Ns MDA mean ± SD 0.20 ± 0.77 0.19 ± 0.07 0.20 ± 0.07 0.26 ± 0.12* 0.025
HAI score median (range) 5 (1–11) 5 (1–10) 6 (1–13) 6 (1–13) Ns
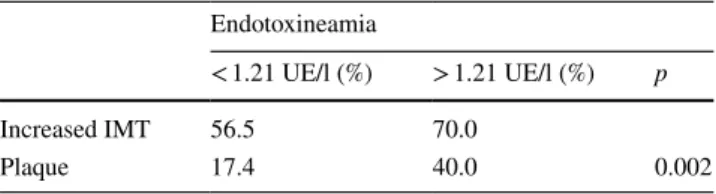
between patients with and without endotoxinemia (Table 1). However, as shown in Table 3, when the data were analyzed using the median value of endotoxinemia as cut-off, a higher prevalence of both increased IMT and plaque were observed in the third and fourth quartiles compared to that observed in the first and second quartiles (p < 0.002). A correlation was also found between IL8 and the presence of atherosclerosis (r = 0.34, p < 0.01).
Factors independently associated with endotoxinemia
An overall analysis of the variable independently associated to the serum endotoxin was evaluated in the multivariate logistic regression model and showed that hepatic steatosis and IL8 were independently associated with endotoxine-mia. However, as seen in Table 4, when the analysis was performed using the highest serum endotoxin levels (4th quartiles), HOMA-IR and MDA also became independent variables.
Discussion
In this study we have shown that, regardless of liver cir-rhosis, endotoxinemia is a condition that occurs with high frequency (60%) in our series of chronic hepatitis C patients and that patients with endotoxinemia, especially those with the highest serum LPS concentrations showed significantly higher levels of pro-inflammatory cytokines and oxidative stress and a significant association with
higher levels of hepatic steatosis, IR and prevalence of atherosclerosis. These data support the concept that by increasing the levels of pro-inflammatory cytokines and oxidative stress, endotoxinemia contributes to the develop-ment of hepatic and extrahepatic manifestations associated with HCV such as fatty liver, IR and atherosclerosis.
In experimental models, LPS has been shown to play a role in the increase of IR, hepatic steatosis and inflam-mation [16]. Most of the biological effects of LPS have been related to the release of pro-inflammatory cytokines, such as TNF-α, interleukins (IL-1, IL-6, IL-8 and IL-12), chemokines (MPC-1) and oxidative stress [17]. Kupffer’s liver cells, through their receptors for LPS, toll-like recep-tors (TLRs), are the primary cells involved in the LPS response producing inflammatory cytokines [18]. TNF-α, which is the main cytokine induced by LPS-TLR4, is rec-ognized as the main mediator of hepatotoxicity, inflamma-tion and development of IR and steatosis [19].
Endotoxinemia has been reported in patients with NAFLD [5, 6], promoting the development of NASH and a worsening of IR [8]. These effects are at least partly related to the increase in intestinal permeability [20], with subsequent translocation of LPS into the portal vein, activating the inflammatory phenotype of Kupffer cells [21] and promoting an inflammatory response [22, 23]. In particular, among the mediators of inflammation (adipocy-tokines), TNFα plays an important role in the pathogenesis of NAFLD/NASH and IR [24, 25].
The role of the imbalance between TNF-α and adi-ponectin is known in the pathogenesis of IR in chronic hepatitis C [26, 27], and the release of free fatty acids from peripheral sites to the liver could be a fascinating hypothetical mechanism contributing to steatosis and IR development in chronic hepatitis C. Furthermore, it has been shown that endotoxin, associated with HCV core pro-tein, is able to promote inflammation in chronic hepatitis C by activating monocyte/macrophage [28].
Our study showed that endotoxinemia occurs with high frequency in chronic hepatitis C patients and is level-dependent associated with increased levels of TNF-α, which, in turn, contribute to the development of IR and fatty liver deposition. In this study, we were unable to demonstrate an increase in necroinflammatory damage and hepatic fibrosis associated with endotoxinemia. However, in previous experimental and clinical studies, the presence of endotoxinemia has been associated with an increase in necroinflammatory hepatic activity and at an advanced stage of liver disease [2, 21, 29]. Moreover, considering that the role of hepatic steatosis and IR is well-known in the progression of liver disease leading to fibrosis [9], it is therefore possible that endotoxin, modulating steatosis and IR, may contribute to the development of liver fibrosis and type 2 diabetes in the long term.
Table 3 Prevalence of atherosclerosis (increased IMT or plaque) according to the median value of endotoxinemia
Endotoxineamia
< 1.21 UE/l (%) > 1.21 UE/l (%) p
Increased IMT 56.5 70.0
Plaque 17.4 40.0 0.002
Table 4 Independent factors associated with all endotoxinemia using all values* or using the highest values of serum endotoxinemia levels (4th quartile) ** Coefficient B std. error 95% CI p Lower Higher Steatosis* 0.243 0.082 0.080 0.406 0.004 IL8* 0.124 0.064 0.042 0.628 0.022 Homa-IR** 0.030 0.015 0.001 0.059 0.046 MDA** 0.272 0.102 0.069 0.475 0.001
Among the biological responses of endotoxinemia, oxi-dative stress is one of the significant effects implicated in hepatic, cardiovascular and metabolic damage [30]. Oxida-tive stress is a condition observed with high frequency in chronic hepatitis C and contributes to the development and progression of liver injury as well as liver in the fat deposi-tion, IR and cardiovascular damage [9, 13, 31]. Data from this study showed that HCV patients with the highest levels of endotoxinemia showed an increase in levels of MDA-HAS as an expression of chronic oxidative stress. Therefore, by causing oxidative stress, endotoxinemia contributes to the hepatic and metabolic damage associated with chronic hepatitis C and in particular steatosis, IR and cardiovascular damage.
Atherosclerosis is known to be associated with chronic HCV infection [11] and both direct and indirect mechanisms have been proposed [32]. Among the indirect mechanisms, a pro-inflammatory environment has been reported in which pro-inflammatory cytokines and oxidative stress play an important role [15]. LPS through TLR4 has been reported as one of the triggers of the inflammatory pathway in the gen-eration of atherosclerosis [33]. Furthermore, oxidative stress contributes to the inflammatory and metabolic processes that generate and maintain atherosclerosis [34, 35]. In this study, patients with a significant amount of endotoxinemia showed a higher prevalence of carotid atherosclerosis in associa-tion with pro-atherogenic condiassocia-tions such as hepatic stea-tosis, IR, oxidative stress and pro-inflammatory cytokines. Among the latter, in addition to TNF-α, we demonstrated a significant increase in IL-8 but not MPC-1. IL-8 is mainly monocyte and macrophage secreted and its main effect is the recruitment and activation of monocytes and neutrophils in an acute site of inflammation [36]. Furthermore, IL-8 is involved in the regulation of the oxidant gene expression and is highly sensitive to oxidants [37]. The role of oxidants in the regulation of IL-8 and other chemokines has relevance in the field of cardiovascular disease [38]. There is a body of evidence that IL-8 plays an important role in atheroscle-rosis [38]. IL-8 is involved in the adhesion phase of mono-cytes in the early stages of atherosclerosis [39], but it plays a role also in the advanced phase of atherosclerosis [40]. Furthermore, LPS is known to release the pro-inflammatory cytokine IL8 from monocytes. Data from our study indi-cate that in chronic hepatitis C patients, significant levels of endotoxinemia contribute to the development of atheroscle-rosis through increased IL8 secretion as well as by oxidative stress, steatosis and IR.
Treatment with direct antiviral agents (DAAs) against HCV is now able to achieve a sustained virological response (SVR) in more than 95% of patients treated; however, histo-logical liver damage and systemic involvement remain and will probably diminish only over the years. Under experi-mental and clinical conditions, a decrease in endotoxinemia
levels by antibiotic or probiotic treatment has been shown to lead to improvement of the associated conditions [41]. Thus, anti-endotoxin treatment in chronic hepatitis C patients with endotoxinemia could be valuable for improving steatosis, IR and atherosclerosis.
In conclusion, the data from this study indicate that endo-toxinemia in chronic hepatitis C occurs with high frequency and is of clinical relevance, influencing, with cross-reaction mechanisms, IR, systemic inflammation, oxidative stress, hepatic steatosis and atherosclerosis. Control of serum endotoxin levels could be useful also in patients with SVR by DAA, slowing the progression of hepatic and extrahe-patic manifestations and perhaps improving their long-term prognosis.
Compliance with ethical standards Conflict of interest There are none to declare.
References
1. Glaros TG, Chang S, Gilliam EA, Maitra U, Deng H, Li L. Causes and consequences of low grade endotoxemia and inflammatory diseases. Front Biosci. 2013;5:754–65.
2. Gaeta GB, Perna P, Adinolfi LE, et al. Endotoxemia in a series of 104 patients with chronic liver diseases: prevalence and signifi-cance. Digestion. 1982;23:239–44.
3. Pascual S, Such J, Esteban A, et al. Intestinal permeability is increased in patients with advanced cirrhosis. Hepatogastroen-terology. 2003;50:1482–86.
4. Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142:1100–101.
5. Thuy S, Ladurner R, Volynets V, et al. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr. 2008;138:1452–55.
6. Yuan J, Baker SS, Liu W, et al. Endotoxemia unrequired in the pathogenesis of pediatric nonalcoholic steatohepatitis. J Gastro-enterol Hepatol. 2014;29:1292–8.
7. Anderson PD, Mehta NN, Wolfe ML, et al. Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab. 2007;92:2272–279.
8. Boroni Moreira AP, de Cássia Gonçalves Alfenas R. The influence of endotoxemia on the molecular mechanisms of insulin resist-ance. Nutr Hosp. 2012;27:382–90.
9. Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358–364. 10. Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP.
Steatosis and hepatitis C: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586–97. 11. Adinolfi LE, Restivo L, Zampino R, et al. Chronic HCV
infec-tion is a risk of atherosclerosis. Role of HCV and HCV-related steatosis. Atherosclerosis. 2012;221:496–502.
12. Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–99.
13. Vidali M, Tripodi MF, Ivaldi A, et al. Interplay between oxidative stress and hepatic steatosis in the progression of chronic hepatitis C. J Hepatol. 2008;48:399–406.
14. Tsui JI, Whooley MA, Monto A, Seal K, Tien PC, Shlipak M. Association of hepatitis C virus seropositivity with inflammatory markers and heart failure in persons with coronary heart disease: data from the Heart and Soul study. J Card Fail. 2009;15:451–56. 15. Oliveira CP, Kappel CR, Siqueira ER, et al. Effects of hepatitis
C virus on cardiovascular risk in infected patients: a comparative study. Int J Cardiol. 2013;164:221–26.
16. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initi-ates obesity and insulin resistance. Diabetes. 2007;56:1761–72. 17. Sakaguchi S, Furusawa S. Oxidative stress and septic shock:
meta-bolic aspects of oxygen-derived free radicals generated in the liver during endotoxemia. FEMS Pathog Dis. 2006;47:167–77. 18. Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the
emerging view. J Hepatol. 2009;51:212–23.
19. Rabelo F, Oliveira CP, Faintuch J, et al. Pro- and anti-inflam-matory cytokines in steatosis and steatohepatitis. Obes Surg. 2010;20:906–12.
20. Miele L, Valenza V, La Torre G, et al. Increased intestinal perme-ability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–887.
21. Brun P, Castagliuolo I, Pinzani M, Palù G, Martines D. Exposure to bacterial cell wall products triggers an inflammatory phenotype in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G571–8.
22. Schnabl B, Brandl K, Fink M, et al. A TLR4/MD2 fusion protein inhibits LPS-induced pro-inflammatory signaling in hepatic stel-late cells. Biochem Biophys Res Commun. 2008;375:210–14. 23. Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated
dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–85.
24. Ceccarelli S, Panera N, Mina M, et al. LPS-induced TNF-α fac-tor mediates pro-inflammafac-tory and pro-fibrogenic pattern in non-alcoholic fatty liver disease. Oncotarget. 2015;6:41434–52. 25. Ruiz AG, Casafont F, Crespo J, et al.
Lipopolysaccharide-bind-ing protein plasma levels and liver TNF-alpha gene expression in obese patients: evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obes Surg. 2007;17:1374–380.
26. Durante-Mangoni E, Zampino R, Marrone A, et al. Hepatic stea-tosis and insulin resistance are associated with serum imbalance of adiponectin/tumour necrosis factor-alpha in chronic hepatitis C patients. Aliment Pharmacol Ther. 2006;24:1349–457. 27. Ashour E, Samy N, Sayed M, Imam A. The relationship between
serum adiponectin and steatosis in patients with chronic hepatitis C genotype-4. Clin Lab. 2010;56:103–10.
28. Dolganiuc A, Norkina O, Kodys K, et al. Viral and host fac-tors induce macrophage activation and loss of toll-like recep-tor tolerance in chronic HCV infection. Gastroenterology. 2007;133:1627–636.
29. Sandler NG, Koh C, Roque A, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220–230.
30. Sakaguchi S, Furusawa S. Oxidative stress and septic shock. FEMS Immunol Med Microbiol. 2006;47:167–77.
31. González-Gallego J, García-Mediavilla MV, Sánchez-Campos S. Hepatitis C, Virus. Oxidative stress and steatosis: current status and perspectives. Curr Mol Med. 2011;11:373–90.
32. Lonardo A, Nascimbeni F, Maurantonio M, Marrazzo A, Rinaldi L, Adinolfi LE. Non-alcoholic fatty liver disease: evolving para-digms. World J Gastroenterol. 2017;23:6571–92.
33. Suganami T, Tanimoto-Koyama K, Nishida J, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91.
34. Carreau A, Kieda C, Grillon C. Nitric oxide modulates the expres-sion of endothelial cell adheexpres-sion molecules involved in angiogen-esis and leukocyte recruitment. Exp Cell Res. 2011;317:29–41. 35. Xi H, Akishita M, Nagai K, et al. Potent free radical scavenger,
edaravone, suppresses oxidative stress-induced endothelial dam-age and early atherosclerosis. Atherosclerosis. 2007;191:281–89. 36. Remick GD. Interleukin-8. Crit Care Med. 2005;33:s646–s647. 37. De Forg LE, Preston AM, Takeuchi E, Kenney J, Boxer LA,
Remick DG. Regulation of interleukin 8 gene expression by oxi-dant stress. J Biol Chem. 1993;268:568–76.
38. Apostolakis S, Vogiatzi K, Amanatidou V, Spandidos DA. Interleukin 8 and cardiovascular disease. Cardiovasc Res. 2009;84:353–60.
39. Weber KS, von Hundelshausen P, Clark-Lewis I, Weber PC, Weber C. Differential immobilization and hierarchical involvement of chemokines in monocyte arrest and transmigration on inflamed endothelium in shear flow. Eur J Immunol. 1999;29:700–12. 40. Simonini A, Moscucci M, Muller DWM, et al. IL-8 is an
angio-genic factor in human coronary atherectomy tissue. Circulation. 2000;101:1519–26.
41. Ait-Belgnaoui A, Durand H, Cartier C, et al. Prevention of gut leakiness by a probiotic treatment leads to attenuated HPA response to an acute psychological stress in rats. Psychoneuroen-docrinology. 2012;37:1885–95.