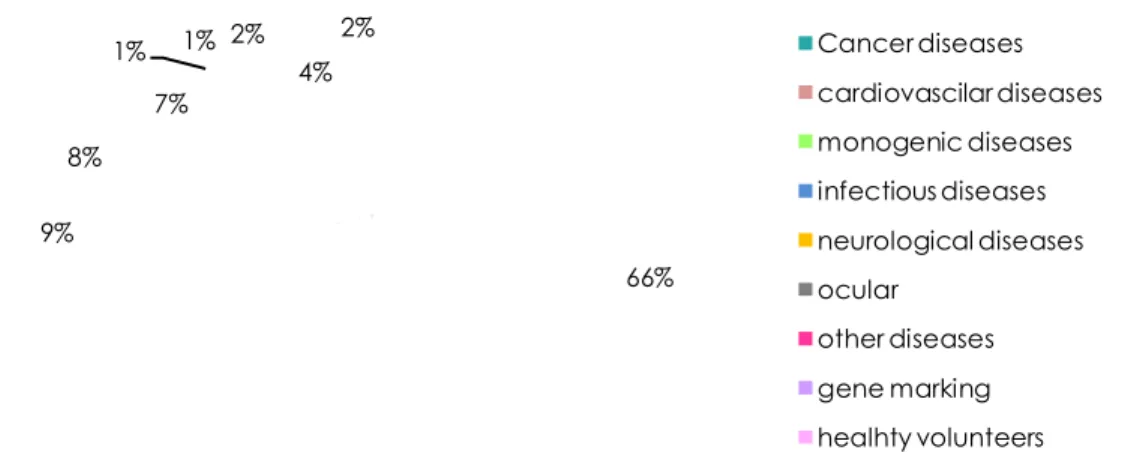1
CHAPTER 1: INTRODUCTION
1.1 BREAST CANCER EPIDEMIOLOGY
Breast cancer is the most commonly diagnosed cancer in women, accounting for 23% of tumors (Parkin et al., 2005). It is estimated that approximately 1 in 11 women will suffer from this disease at some point in their lifetime. Over 200,000 cases were diagnosed in U.S. in 2005, and their incidence continues to rise worldwide with an estimated 1.45 million new cases in 2010 alone (James et al., 2007). Improvement of breast cancer treatments over the last few decades has sensibly reduced overall mortality. However, despite these advances, 30-40% women are diagnosed with metastatic cancer or develop metastases and eventually succumb (James et al., 2007).
Over 50% cases are in industrialized countries, about 361,000 in Europe (27.3% of cancers in women) and 230,000 in North America (31.3%). Incidence rates are high in most developed areas with the highest age-standardized incidence in North America (Jemal et al., 2004) (Fig 1.1). In contrast, breast cancer occurs less frequently in Eastern Europe, South America, Southern Africa, and Western Asia, but still ranks first among female tumors. Rates are low (<30 per 100,000) in Africa (except South Africa) and Asia (Parkin et al., 2005). The highest incidence in developed contries is probably due to accurate and endorsed screening programs to detect the tumor in early stages.
Another possible factor accounting for the different incidence in the world is age. Breast cancer increases with aging. It is rare between 20 and 30 years and sharply increases at 30-45 years. Incidence then plateau at menopause to slowly increase again in postmenopausal age (Benz, 2008).
2 Figure 1.1: Worldwide rates of breast cancer incidence and mortality (Adapted from Parkin et al, 2005.)
3
1.2 RISK FACTORS
Breast cancer onset is correlated with genetic, biological and environmental factors. Several studies demonstrated a positive correlation between estrogens and progesterone concentration and breast cancer. Estrogens are important to stimulate growth of a large proportion of breast cancers. Progesterone has an active role in breast development and tumorigenesis (Russo & Russo, 2006). Hormonal therapies (tamoxifen, anti-estrogens) and adjuvant chemotherapy (Herceptin) have benefited millions of patients with breast cancer. However their success is restricted to a subset of patients whose tumors express specific hormonal receptors such as estrogen receptor alpha, progesterone receptor and c-erbB2 gene (Hussein et al., 2008). Possible correlations between oral contraceptives (OCs) consumption and cancer are still under scrutiny. If, on one hand, OCs appear to be effective in reducing the risk of epithelial ovarian carcinoma (Ferretti et al., 2007), their role in breast cancer is still controversial (Gill et al., 2006) (Yankaskas, 2005). Reduced risk of cancer has been observed in women who had children compared to nullipara (Jacobson et al., 2008). Other studies showed that risk is reduced by 7% for each baby a woman had and further reduced of 4.3% for every 12 months she breast feeded, also intermittently (Woodman, 2002). Since breast is particularly susceptible to carcinogen exposure up to first full-term pregnancy, early age of first full-term pregnancy could be a protective factor (Sivaraman & Medina, 2002). Diet is a modifiable risk factor. Fruits and vegetables, low-fat dairy products, fish, mono-and poly-unsaturated fatty acids, vitamin D, calcium, and phytoestrogens may reduce incidence. In contrast, high intake of meat, poultry, total energy, total fat and saturated fatty acids may play a causative role in this disease (Bissonauth et al., 2008). A significant correlation between breast cancer and alcohol intake has also been observed (Berstad et al., 2008), whereas no associations were found with cigarettes smoking (Rollison et al., 2008) (Lin et al., 2008).
Environmental factors are known to be involved in cancer trigger or development. For instance, some environmental and/or lifestyle factors shift breast cancer incidence among migrant Asian women from baseline rate in their native country to their adopted country (Matsuno et al., 2007). Experimental studies carried out in animal models have identified potential carcinogens in the environment. These chemicals include halogenated, components of gasoline, aromatic amino/nitro compounds, dyes, epoxides, products of combustion common industrial solvents
4 and pesticides (Brody & Rudel, 2003). They act by mimicking or disrupting hormones that promote or inhibit tumor growth. Many of these chemicals have been shown to mimic estrogens in vitro by binding estrogen receptor, initiate transcription of estrogen-regulated genes, and stimulate breast cancer cells (Laws et al., 2000). Ionizing radiation has been firmly established as an environmental cause of breast cancer. Studies of atomic bomb survivors and women exposed to X-ray medical treatments in childhood indicate that exposures early in life impart greater risk than adult exposures. Studies of exposed Japanese women carried out 35 years after the atomic bomb, demonstrated that risk of breast cancer was 4-fold greater in women younger than 4 years of age and 2-fold greater in women 10–14 years compared with women 20–30 years of age at time of bombing. Women under forty had a greater risk than those older than 40 at time of bombing (Preston et al., 2007).
1.3 HEREDITARY BREAST CANCER
Epidemiological studies demonstrated that the risk of developing breast cancer is a 2-3 fold higher in blood relatives of a cancer patient compared to the rest of population (Albrektsen et al., 2006). This risk is higher if more members of a family are diagnosed with cancer. Such a high risk must be related with a complex genetic mechanism. A study carried out in 1994 confirmed the hypothesis of a hereditary genetic mechanism (Claus et al., 1994) and led to the identification of two genes conferring breast cancer susceptibility: BRCA1 and 2 (Miki et al., 1994).
Today is known that 90% of breast cancer cases are sporadic, whereas up to 10% are likely due to germ-line mutations in specific genes (Teng et al., 2008). In particular, 80% hereditary breast cancers are associated to BRCA1 or BRCA2 mutation(s) (fig 1.2). Moreover, risk increases to 60%-80% in women carrying BRCA mutations (Teng et al., 2008). Percentage of cases due to germ-line mutations is higher in women developing neoplasia under 35 years of age and is around 35% (Lux et al., 2006). Although BRCA1 gene mutations are rare in sporadic breast cancers, BRCA1 expression is often reduced or absent in sporadic cases suggesting a much wider role in both hereditary and sporadic mammary carcinogenesis (McCoy et al., 2003). Why mutations of this gene strongly predispose to breast and ovarian cancers remains controversial.
5 Figure 1.2: Factors promoting breast cancer.
1.4 BRCA1 GENE AND FUNCTIONAL DOMAINS
BRCA1 was initially located to chromosome 17 by genetic linkage analysis in 23 early-onset breast cancer family (Hall et al., 1990) and cloned and isolated in 1994 (Miki et al., 1994). BRCA was later localized to 17q21 and is 100 kb long (fig 1.3). BRCA1 has 24 exons, including 2 untranslated exons, and encodes a protein of 1863 amino acids (aa). BRCA1 also encodes for at least two other smaller proteins originated by alternative splicing. One variant, BRCA1-∆11, is identical to the full-length form except for the absence of exon 11. As the nuclear localization signal (NLS) is within this exon, BRCA1-∆11 localizes in the cytoplasm (Kim et al., 2006). The other variant is BRCA1-IRIS, which is a 1399-residue polypeptide encoded by an uninterrupted open reading frame (ORF) that extends from codon 1 of BRCA1 ORF to a termination point 34 triplets into intron 11 (ElShamy & Livingston, 2004). Full-length BRCA1 contains
BREAST CANCER
HEREDITARY SPORADIC 5-10% 90-95% 80% 20% 33% BRCA1 (40-45%) BRCA2 (35-40%) mutation P53/PTEN ATM/BRCA3 ? mutation BRCA1 decreased expresssion6 multiple functional domains; the amino terminal (N-terminal) is characterized by a zinc-binding RING-finger (8-96 aa) domain formed by a conservative motif containing 7 cysteins and 1 histidine. This motif is known to be responsible for BRCA1 interaction with BRCA1-associated RING domain 1 (BARD1) and BRCA1-associated protein (BAP1). RING finger is also part of a larger motif involved in E3 ubiquitin ligase activity. Region included between aa 200 and 300 contains 2 NLS and sites of interaction with proteins involved in cell cycle checkpoint such as p53 and RB, in transcription regulation (cMyc, ZBRK, GADD45) and factors facilitating transcription by altering the chromatin structure (SW1/SNF). BRCA1 central portion contains a DNA binding domain used for the interaction with RAD50, a protein known to be involved in DNA repair, and BRCA1 associated surveillance complex (BASC) which plays a role in cell cycle checkpoint. From aa 1,280 to 1,524 the SQ-cluster domain (SCD) is located.
Figure 1.3: Localization of BRCA1 gene (from
7 This serine and threonine cluster is phosphorilated by several kinases involved in cell cycle regulation such as ATM, CHK2 and CDK2. BRCA1 carboxyl-terminal (C-terminal) contains 2 tandem copies of a highly conserved 95 aa long domain known as BRCT domain. Structural studies highlighted a relatively conserved structure of two or three α helices surrounding a central β-sheet.
BRCT domains are present in many other proteins besides BRCA1 and seem to facilitate physical interactions among proteins involved in the cellular response to DNA damage (Caldecott, 2003). Through BRCT motif BRCA1 binds to RNA polymerase II, p300, p53, BACH1, CtIP, HDCA1 and 2. This interaction is responsible for BRCA1 role in cell cycle regulation and DNA repair (fig. 1.4) (Deng, 2006).
Figure 1.4: Functional domains of BRCA1 (Adapted from www.nature.com/nrc/journal/v4/n9/abs/nrc1431.html).
8
1.5 ROLE OF BRCA1 IN CELLULAR HOMEOSTASIS
Current studies suggest that multiple functions of BRCA1 may contribute to tumor suppressor activity, including roles in cell cycle checkpoints, transcription, protein ubiquitination, apoptosis and DNA repair.
1.5.1 DNA REPAIR
BRCA1 plays a major role in the cellular response to DNA damages by protecting cells from double strand break (DSB) that arise during DNA replication or after DNA damage (Zhang & Powell, 2005).
There are two major types of DSB repair: homologous recombination (HR) and non-homologous end-joining (NHEJ). Homologous recombination is typically free from mistakes, because an extensive non-damaged homologous sequence is used to repair the damaged duplex by gene conversion. NHEJ uses no sequence homology and usually involves a change in sequence at the break point. Perhaps, more importantly, the extent of sequence modification varies from loss or gain of 1 or 2 bp to large-scale deletions. The mutagenic potential of this pathway is determined as much by the quality of the repair rather than just whether the break is rejoined. If there is loss of sequence at the site of the break, the resulting junction is often mediated by a region of micro homology, up to 8 bp in size. (Richardson & Jasin, 2000).
After ionizing radiation, mediator of DNA damage checkpoint protein 1 (MDC1) can regulate BRCA1 to the sites of DNA lesions and phosphorylate it through ataxia telangiectasia mutated (ATM) dependent pathways. Following activation BRCA1 can bind to p53, RAD50-MRE11-NSB1 (R-M-N) complex and RAD51, conducting HR or NHEJ (fig.1.5) (Teng et al., 2008). R-M-N complex localises in DSB region and through its 5’-3’exonuclease exposes the 3’ ends of damaged DNA (Hoeijmakers, 2001). The break is then repaired by one of two homologous repair systems: single-strand annealing, which uses regions of homology between the complementary strands to align the strands of DNA and strand invasion, which uses the sister chromatid as a template to repair the break. BRCA1 appears to participate in strand invasion by forming a complex with BRCA2, RAD51, and proliferating cell nuclear antigen at regions of DNA damage (Kennedy et al., 2004). NHEJ is used for the majority of DNA
9 breaks in mammalian cells and is active throughout the cell cycle. BRCA1 is causally linked to in vitro and in vivo NHEJ activity probably due to its ability to regulate microhomology in NHEJ. Although its correct role is still controversial (Baldeyron et al., 2002) it is known that in BRCA1 absence, DNA repair may be DNA less precise and, therefore, potentially more harmful (Kennedy et al., 2004). Thus, in the absence of BRCA1 or in presence of a mutated form of the protein, HR is deficient and NHEJ may become even less accurate resulting in defective repair that may increase the toxicity of DNA damage.
1.5.2 CELL CYCLE CHECKPOINTS
The ability to control precisely the ordering and timing of cell cycle events is essential to maintain genome integrity and prevent mutations that disrupt normal growth control. BRCA1 has been implicated in regulation of cell cycle checkpoints (fig.1.5). The mechanisms through which BRCA1 regulate various cell cycle checkpoints may be related, at least in part, to the finding that BRCA1 is a substrate for the so-called DNA damage response kinases, including ATM, ATM-related kinase (ATR), and cell cycle checkpoint kinase 2 (CHK2). ATM is activated by the type of double-strand DNA breaks that are caused by ionizing radiation, whereas ATR is activated by stalled DNA replication forks (Zou & Elledge, 2003). These DNA damage response kinases appear to have both overlapping and distinct phosphorylation sites on BRCA1 which is phosphorylated by the ATM kinase on serines 1387, 1423, 1457, and 1524 in response to ionizing radiation (Gatei et al., 2001), by CHK2 on serine 988 (Zhang et al., 2004) in response to ionizing radiation and by ATR on serine 1423 in response to UV radiation (Tibbetts et al., 2000). Phosphorylation of BRCA1 on serine 1423 is required to activate the G2/M-phase checkpoint, whereas activation of the intra–S-phase checkpoint requires phosphorylation on serine 1387 (Xu et al., 2002). The mechanism used by phosphorylated BRCA1 to modify the activity of the various checkpoints is still unclear, but it may be partly associated with the transcriptional activation of key checkpoint proteins. BRCA1 apparently stimulates the transcription of the p21 and p27 genes, which arrest cells at the G1/S-phase boundary and in S phase, respectively, by inhibiting cyclin-dependent kinase 2 (Park et al., 2008). BRCA1 also stimulates the transcription of GADD45, which can activate the G2/M-phase checkpoint by binding to the cyclin B–cdc2 complex and preventing its
10 localization to the nucleus (Park et al., 2008). BRCA1 represses both the expression of cyclin B, also thereby activating the G2/M-phase checkpoint (Yarden et al., 2002 and the polo-like kinase (PLK) gene, which encodes a protein required for progression into mitosis {Ree, 2003 #47). Therefore, BRCA1 can function at multiple levels to regulate the G2/M-phase and G1/S checkpoint and appears to be essential in promoting DNA repair and prevent replication of damaged DNA by regulating cell cycle progression.
Figure 1.5: Functions and cellular factors interacting with BRCA1 (Adapted from www.nature.com/nrc/journal/v4/n9/abs/nrc1431.html).
11
1.5.3 UBIQUITINATION ACITVITY
BRCA1 is an ubiquitin E3 ligase (Baer & Ludwig, 2002). It has been recently reported that polyubiquitin chains recruit BRCA1 to damaged DNA sites (Kim et al., 2007), suggesting that ubiquitination plays intervenes upstream and downstream the BRCA1 pathways. The ubiquitin system is made of three enzymes: a ubiquitin-activating enzyme (E1), a ubiquitin-conjugating enzyme (E2), and a ubiquitin ligase (E3). The E3 catalyzes the formation of polyubiquitin chains (or sometimes monoubiquitin), using ubiquitins activated by the E1 and E2 enzymes, and transfers them onto specific substrate(s) via isopeptide bonds. As mentioned in Section 1.4, BRCA1 contains an N-terminal RING finger domain, a common E2 binding motif found in E3 ligases, and tandem BRCT domains in its C-terminal region. The N-terminal RING finger domain of BRCA1 interacts with another conformationally similar RING finger protein, BARD1, that contains an N-terminal RING domain and C-terminal tandem BRCT domains to form a RING heterodimer E3 ligase (Baer & Ludwig, 2002) (Brzovic et al., 2003). BRCA1-BARD1 likely acts like a E3 in many cellular complexes and directs the ubiquitination of distinct substrates within each complex. Its putative substrates identified at present are histones, γ-tubulin Erα, nucleophosmin/B23, the largest subunit of RNA polymerase II, CtIP, the common subunit of RNA polymerases, progesterone receptor-A, and the general transcription factor TFIIE (Wu et al., 2008) Biological consequences for the ubiquitination of the above substrates are not yet completely understood.
One of the reasons for the incomplete knowledge is that the signal mediated by BRCA1-induced ubiquitination has not been determined. Depending upon the type of ubiquitin chain, ubiquitin modifications signal a variety of processes. These processes also need to be defined. On the other hand, the ubiquitination-dependent BRCA1 recruiting mechanism after DSB has been elucidated and γH2AX polyubiquitination, RNF8, Ubc13, RAP80, and now it is known that ABRA1 are involved in the system. Turnover of polyubiquitinated structures by DUB activity, such as by BRCC36 or BAP1, may further regulate this reaction (Wu et al., 2008)
12
1.6 BRCA1 INACTIVATION MECHANISMS AND CONSEQUENCES
BRCA1 is highly polymorphic with more than 1,200 distinct documented variants and information regarding their association to cancer is virtually absent for a significant proportion of them (Breast Cancer Information Core (BIC) Database: http://www.nhgri.nih.gov/Intramural_research/Lab_transfer/Bic/) (Szabo et al., 2004). One explanation of the high BRCA1 sequence variability is that several regions of the protein have little structural constraints and can therefore tolerate amino acid changes without significantly affecting function. Functional mutations are heterogeneously localized in the entire BRCA1 gene and more often in coding region and intronic sequences along the exons. Most mutations are frameshift or nonsense, causing premature termination of nascent protein (Thomassen et al., 2008) (Szabo et al., 2004) but chromosomal rearrangements and regulatory mutations have also been described (Fig. 1.6). The tumor suppressive function of BRCA1 appears highly sensitive to loss of the carboxy-terminal region in fact most of known BRCA1 nonsense or frameshift mutations are localized in these regions such as the frameshift 5382insC and the nonsense Y1853X that, although causing the deletion of only 11 aminoacids from the 1863-residue protein, has been clearly defined by genetic studies to be cancer-associated (Friedman et al., 1994).
13 The functional consequences of missense variants, often localized in functional domains or in splicing sites, are still difficult to predict. It is presumable that affecting protein tertiary structure, they may disrupt protein associations leading to deregulation of cellular functions and eventual progression to malignancy. Some of the missense mutations are located in two BRCA1 motifs: the BRCT domain and the RING finger domain, and some others are located in regions of interaction with proteins relevant for its function such as RAD50 and RAD51, suggesting that the BRCA1 protein structure in those regions is critical (Figge & Blankenship, 2004) (Sauer & Andrulis, 2005).
Cells lacking BRCA1 also exhibit chromosome aberrations such as whole chromosome duplications and translocations (Skibbens et al., 2008). Chromosomal rearrangements have been found to be major founder mutations in some ethnic groups, accounting for a significant proportion of BRCA1 mutations in the Dutch population (Szabo et al., 2004). These mutations are thought to be increased by the high homologous recombination frequence between Alu repeated sequences in intronic regions (Agarwal et al., 2006). It has been proposed that these sequences might also promote deletion of chromatin loops containing large portions of the BRCA1 gene. Regulatory mutations are a broad category that includes nucleotide substitutions in regulatory regions causing altered gene expression. Similar outcome is also expected from mutations in regions important for mRNA stability or translation efficiency. Promoter hypermethylation has been reported to occur somatically, and may account for loss of BRCA1 protein expression in sporadic breast carcinomas (Rice et al., 2000). Regardless of type of mutation, BRCA1 inactivation leads relentlessly to serious consequences. In most cases to breast or ovarian cancer, but recently it was also associated to Fanconi Anemia.
1.6.1 BREAST CANCER
Hereditary breast cancers arising in people carrying BRCA1 and/or BRCA2 mutated genes differ from sporadic breast cancers in their morphological, immunophenotypic and molecular features. BRCA1 associated breast carcinomas occur at a very early age (James et al., 2007) and patients are often premenopausal and have positive lymph nodes (Veronesi et al., 2005).
14 In general, BRCA1 associated carcinomas are infiltrant ductal carcinomas of high grade (usually Grade 3) (Teng et al., 2008). They have the “basal-like” phenotype characterized by expression of basal or myoepithelial markers such as basal keratins (CK5/14/17), high mitotic count, negative Her2 and estrogen receptor (ER) status and distinct gene expression signature (Jumppanen et al., 2007). BRCA1 associated breast carcinomas usually have also the over-expressions of cell-cycle proteins such as cyclins A, B1 and E, and S-phase kinase-associated protein 2 (SKP2) (Honrado et al., 2005).
1.6.2 FANCONI ANEMIA
Fanconi anemia (FA) is a rare autosomal or X-linked recessive disease characterized by chromosomal instability and cancer susceptibility. Typically, FA patients develop bone marrow failure leading to aplastic anemia during the first decade of life and at least 20% develop malignancies. Most commonly, these include acute myelogenous leukemia and myelodysplastic syndrome, but also head and neck squamous cell carcinoma, esophageal carcinoma, and liver, brain, skin and renal tumors (Alter, 2003) (Hoskins et al., 2008).
All FA proteins are required for cellular resistance to DNA crosslinking agents and are considered to cooperate in a common pathway (the FA pathway) that regulates the sensing, signalling and/or repair of interstrand DNA crosslinks. FA proteins are closely related to BRCA1 and BRCA2, and to their partner proteins. FANCD1 protein is identical to BRCA2; FANCJ is identical to BACH1/BRIP1, a DNA helicase that interacts directly with BRCA1. Furthermore, some of these proteins form a core complex that is involved in the monoubiquitination and interacts with BRCA1. In light of these interplays between FA and BRCA proteins, the FA pathway is also called the “FA-BRCA pathway” or “FA-“FA-BRCA network”.
1.7 HEREDITARY BREAST CANCER PREVENTION
As mentioned in Section 1.3, Individuals with deleterious mutations in BRCA1 and BRCA2 genes have an estimated risk of breast cancer between 50% and 80%.Such individuals are easily identified as candidates for breast cancer prevention and
15 screening strategies. Multiple retrospective and prospective studies have established that prophylactic mastectomy is the most effective strategy for the prevention of breast cancer in high-risk women. One of the first studies conducted in 1999 demonstrated 80%-100% reduction in breast cancer following prophylactic mastectomy in high and moderate-risk women, respectively, with similar findings restricted to a small series of BRCA1 and BRCA2 mutation carriers (Hartmann et al., 1999). In a larger study carried out in 2004, 483 BRCA1/2 carriers who chose mastectomy or surveillance were examined. At a mean follow-up of 6.4 years, there were two cases of breast cancer in the 105 women choosing mastectomy compared with 184 among 378 mutation carriers choosing surveillance, corresponding to a risk reduction of approximately 95% (Rebbeck et al., 2004). Although prophylactic mastectomy is highly effective, its benefits must be weighed against its potential complications and effects on quality of life. In quality-of-life studies, most women who have undergone prophylactic mastectomy are satisfied with their decision (Frost et al., 2000). However the uptake of prophylactic mastectomy is low; in most series, less than 30% of BRCA1 or BRCA2 mutation carriers choose such surgery (Phillips et al., 2006) (Uyei et al., 2006). Alternatively, albeit less effective, other strategies to reduce breast cancer risk are prophylactic oophorectomy and chemoprevention.
Several studies showed that oophorectomy in BRCA1/2 carriers, significantly reduces the risk of ovarian cancer and breast cancer by 50% or more (Rutter et al., 2003), (Kramer et al., 2005). Data from Domchek et al. have suggested that prophylactic oophorectomy may improve overall survival and cancer-specific survival in women with BRCA1/2 mutations. At a median follow-up of 2 to 3 years, prophylactic oophorectomy led to a risk reduction for breast and ovarian cancer consistent with prior reports. In addition, a reduction in breast cancer specific mortality, ovarian cancer–specific mortality, and overall mortality were also seen (Domchek et al., 2006). Although prophylactic oophorectomy is associated with a low risk of operative complications, and despite the multitude of benefits associated with this procedure, some women delay or forego prophylactic oophorectomy due to fear of symptoms related to surgical induced menopause (Rodriquez & Domchek, 2007). However, recent data suggest that short-term hormone replacement therapy (HRT) after prophylactic oophorectomy in premenopausal women with no prior history of breast cancer does not significantly influence breast cancer risk. Anyway, whether
short-16 term HRT following prophylactic oophorectomy prevents long-term complications such as osteoporosis or ischemia heart disease is not known.
As regards chemoprevention, Tamoxifen, the world largest selling drug for breast cancer treatment is an orally active selective estrogen receptor modulator. It has been demonstrated the effectiveness of tamoxifen in reducing breast cancer risk among healthy women and the decrement of invasive and non-invasive breast cancer incidence for all women, including those with a family history (Rodriquez & Domchek, 2007). There are several reasons to believe that BRCA1 associated breast cancer risk could be reduced by blocking the activity of endogenous estrogens: the incidence of breast cancer decline after 50 years of age, pregnancy (during which estrogens concentration heighten) increases the risk of early-onset breast cancer and premenopausal oophorectomy in BRCA1 mutation carriers reduce the risk (Narod et al., 2000). This seems to be connected to a probable BRCA1 role in reduction of proliferative response of the breast epithelium during period of estrogens exposure. In this context tamoxifen might reduce breast cancer risk through mechanism such as receptor-mediated estrogens blockade.
1.8 GENE THERAPY
Preventive mastectomy is an invasive procedure and sometimes it is responsible for serious psychological and social consequences related to self-esteem, satisfaction with body appearance,feelings of femininity and sexual relationships. Tamoxifen, and chemoprevention in general, cause heavy side effects ranging from menopausal symptoms to an increased risk of developing endometrial cancer (Narod et al., 2000). On these premises the development of novel preventive strategies to treat BRCA1 mutations carriers are essential. Patients with a hereditary breast and ovarian cancer due to BRCA1 mutations are born with a mutation on one BRCA1 allele, but they develop cancer only after mutation or allelic loss of the other BRCA1 allele (Obermiller et al., 2000).
As mutational inactivation of BRCA1 gene function is the main trigger of carcinogenesis, gene correction strategies may provide an opportunity for selective targeting without significant toxicity for normal non tumor cells.
In vivo gene transfer aims to insert genetic material into an organism using a vector that would transfer only the required gene to de desired target cells; the ultimate
17 goal of gene transfer is gene therapy whose purpose is to use genes as therapeutic units.
Gene therapy, in fact, is a novel approach aimed to deliver genetic information to a target cell, by either replacing a defective gene or introducing an additional function to treat or prevent a disease. On these premises, replacing mutated BRCA1 allele into specific mammalian cells that bear a BRCA1 mutation but have not undergone full transformation yet could represent a new preventative strategy against breast cancer. In most gene therapy studies, a correct copy or wild type gene is provided or inserted into the genome. Generally, it is not an exact replacement of the “abnormal,” disease-causing gene, but rather extra, correct copies of genes are provided to complement the loss of function. A carrier known as vector must be used to deliver the therapeutic gene to the target cells.
1.9 AVAILABLE TOOLS FOR IN VIVO GENE DELIVERY
Currently, there are two common ways of performing gene delivery into desired
cells: non-viral techniques or virus-derived vectors. The most common non-viral method to perform gene delivery is the use of liposomes that presents certain advantages over viral methods such as simple large-scale production and low host immunogenicity. Moreover, in delivering genes to cells they can complex both with negatively and positively charged molecules; they offer a degree of protection to the DNA from degradative processes and they can carry large pieces of DNA, potentially as large as a chromosome. Anyway, low levels of transfection and expression of the gene remain a great disadvantage and for this reason, viral vectors are the most used tool to perform gene delivery so far.
1.9.1 SUITABLE VIRAL VECTORS
The lack of efficient, non-toxic, gene delivery systems, rather than the paucity of therapeutic genes, is the major challenge of in vivo gene therapy. Using vectors derived from viruses as ‘Trojan horses’ to reach the required cells means taking advantage of millions of years of evolution. (Bouard et al., 2008). In fact, viruses are one of the most efficient tools to perform gene delivery into specific target cells not
18 only for their specific targeting but also because they are naturally very efficient at inserting their own genetic information into host cells for their own replication.
By replacing nonessential viral genes with foreign genes of therapeutic interest, recombinant viral vectors can be used to transduce the cell type that they would normally infect or, using specific a heterologous glycoprotein (GP), different cell types.
One of the major concerns in viral vector design and production is biosafety and vector security. Vectors and cell based vector production systems have to be approved by regulatory agencies (Bouard et al., 2008). Nowadays viral vectors tend to be devoid of as many viral sequences as possible, to be non-pathogenic or replication-defective, and therefore they are likely to be of low toxicity.
To date, up to 1000 clinical gene therapy trials have been initiated using a limited number of viruses as vectors, namely adenoviruses (Ad), retroviruses (specifically lentiviruses), adeno-associated viruses (AAV) and herpes simplex virus (HSV). The main indication addressed by these clinical trials is cancer, followed by cardiovascular and monogenic diseases (fig. 1.7).
Figure 1.7: Ongoing gene therapy trials.
66% 9% 8% 7% 1% 1% 2% 4% 2% Cancer diseases cardiovascilar diseases monogenic diseases infectious diseases neurological diseases ocular other diseases gene marking healhty volunteers
19
Ad vectors
Ad virions consist of a ~26-40 kb linear dsDNA genome encased within a non-enveloped icosahedral particle. Since their discovery in 1953, over 50 different serotypes of human adenoviruses have been isolated and characterized and the family Adenoviridae has been shown to be comprised of numerous non-human serotypes from a variety of mammalian, avian, reptilian, amphibian, and even fish species (Davison et al., 2003). Among this wide variety of serotypes, number 5 and 2 are the most used to produce Ad vectors
Vectors based on Ad5 can mediate high levels of transduction in a wide variety of both quiescent and proliferating cells. Transgene expression from Ad5 vectors is typically transient because the Ad5 genome does not integrate into host cell chromosomes. First-generation replication deficient Ad5 vectors have been developed by the deletion of the El genes, necessary for expression of E2 and late genes required for Ad DNA synthesis, capsid protein expression, and viral replication. E1 was provided in trans by the cells in which the vector was produced (Graham & Prevec, 1995).
Subsequent deletions included E3 genes involved in anti-host immunity that are dispensable for viral replication in vitro. Deletion of El and E3 regions creates room for about 8 kb of foreign DNA to be inserted into the Ad vector genome, thus permitting the expression of transgenes in mammalian cells infected with the Ad vector (Campos & Barry, 2007). Third generation Ad vector (gutted vectors) contain only viral terminal repeat and encapsidation signal and are produced in a cell line expressing E1and in presence of a virus helper that express all the other viral proteins. One of the first barriers that gene therapy vectors have to circumvent in vivo is the immune response, in particular the complement system and other components of innate immunity as well as pre-existing antibody-mediated immunity. The Ad vectors were initially susceptible to immune system attack (complement system and pre-existing antibodies) but progress was made by engineering ‘gutted’ or ‘helper-dependent’ vectors stripped of all viral genes, thereby limiting the production of potentially harmful viral antigens. Anyway, the problem remains and sometimes has led to heavy consequences. An extreme example occurred to a patient with ornithine transcarbamylase deficiency who died of systemic inflammatory response syndrome after hepatic arterial injection of an Ad vector (Raper et al., 2003)
20
AAV vectors
AAV is a small, icosaedral and nonenveloped virus belonging to the parvoviridae family. Productive infection and replication requires a helper virus, such as Ad or HSV. In the absence of a helper virus, AAVs establish a latent infection within the cell, either by site-specific integration into the host genome or by persisting in episomal forms.
Interest in AAV vectors is due to virus nonpathogenicity and nonimmunogenicity as well as its heat stability and resistance to solvents and to changes in pH and temperature (Wright et al., 2003). Moreover, they retain only about 300 nucleotides of viral sequence, which greatly improve safety for human clinical applications, have a broad host and cell type tropism range, and transduce both dividing and nondividing cells in vitro and in vivo. However, their size limits the insertion of gene expression cassettes that cannot be greater than 5 kb. This problem and the slow gene expression, due to the requirement of conversion of the single-stranded AAV DNA into doublestranded DNA before gene expression can be initiated, make them poorly suitable for in vivo gene delivery (Coura Rdos & Nardi, 2007)
HSV vectors
HSV is a neurotropic DNA virus. The viral genome consists of 152 kb of dsDNA and encodes 84 viral genes that can be divided into essential and nonessential genes according to their dispensability for viral replication in vitro. Nonessential genes may be deleted in HSV vectors thus allowing insertion of exogenous genetic material. This aspect connected to virus high infectiveness, capability of transducing non-dividing cells, and the latent behaviour, makes HSV very attractive when considering the design of gene therapy vectors. HSV vectors can be obtained in two different ways: cloning the transgene in a plasmid containing HSV origin and encapsidation signal which is then transfected in a cell line infected with a HSV helper virus; or cloning the transgene in a plasmid alongside specific HSV sequences and co-transfecting it with HSV in cell lines. Regardless of procedure, homologous recombination leads to transgene insertion into HSV.
Due to their capability of establish latency in neuronal cells, HSV vectors have been tested for neurological diseases treatment. Moreover they have been used also to develop therapies against cancer and rheumatoid arthritis (Burton et al., 2001).
21 Like Ad, HSV vectors are subjected to pre-existing immunity. Furthermore, difficulties in obtaining a long-term transgene expression in specific areas, and hitches related to vector realising in host cells have reduced their application so far.
Retroviral vectors
Retroviruses are enveloped viruses with a capsid containing a single copy of positive sense RNA of 7-11 kb. Once entered into host cell RNA genome is reverse transcribed in a double helix DNA through the reverse transcriptase and then integrated into the host’s genome, at which point the retroviral DNA is referred to as a provirus.
The latter capability makes retroviruses very attractive for gene therapy because integration facilitates long-term transgene expression. Several retroviral vectors have been developed from Murine Leukemia Virus (MLV). MLV genome, as all retrovirus genomes, has viral promoters known as long terminal repeats (LTR) at 5’ and 3’ends. Three large open reading frames (ORF) are positioned alongside LTRs. These ORFs, known as gag, pol and env, encode structural proteins, enzymes and surface GPs respectively. The collocation of LTRs and other cis-acting elements (such as encapsidation signal (ψ)) in terminal regions has made possible the deletion of large portions of provirus genome and the insertion of exogenous DNA up to 8 Kb.
Today replication-deficient vectors are the most commonly used. In fact, most of the protein-coding regions of the virus genome are removed and replaced with the transgene. The missing protein functions are provided in trans, either transiently or from pre-integrated constructs in appropriately engineered packaging cells. Whilst virion may infect cells, resulting in integration of the provirus in the DNA of the new host, the genes encoding the packaging proteins normally will not be transferred. Consequently, no further replication of the vector occurs and the virus will not spread beyond the progeny of the originally infected cell (Dong et al., 2008). A MLV vector has been the first engineered virus to be tested in a clinical trial to deliver adenosine deaminase to correct severe combined immunodeficiency (Blaese et al., 1995) with encouraging results. Although MLV derived vectors are characterised by relatively low viral titre, difficulties in vivo administration and low transduction efficiency (Wysocki et al., 2001). This last problem is connected, in part, to the incapability of retroviruses to pass through cellular membrane and they can infect only dividing cells whose membrane breaks up during mitosis. Therefore, they can be used only in specific cases such as cancer treatment where cells divide quickly but they are less useful in pathologies involving quiescent cells.
22
Lentiviral vectors
Lentiviruses share many characteristics with retroviruses but have evolved cis and trans-acting factors that allow active transport of the DNA to the nucleus of non-dividing cells. This process facilitates infection of quiescent cells and, in the case of lentiviral vectors, allows transduction of hematopoietic cells, neurons or immunity system cells and all the other cells that rarely divide. For this reason engineered lentiviruses are nowadays the most commonly used tools to set up gene therapy protocols. One of the first lentiviral vector model was developed from human immunodeficiency virus-1 (HIV-1) in 1996 (Naldini et al., 1996) and proved able to transduce in vivo non-dividing human cells.
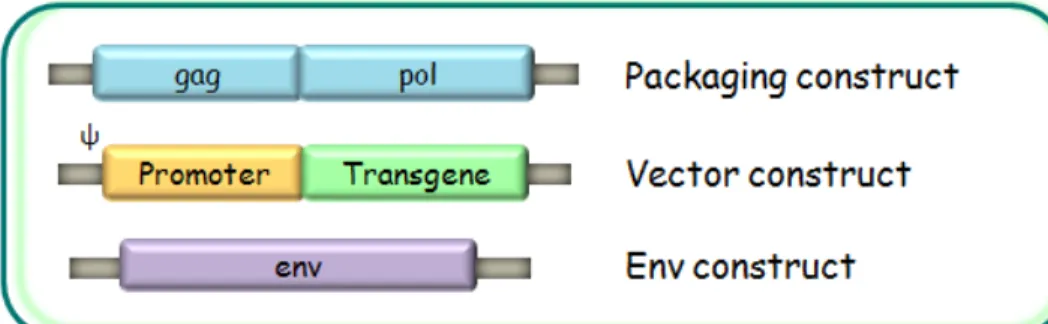
Generally, HIV-1 derived vectors are developed following a split-component system in which cis-acting sequences necessary for viral genome transfer into host cells are divided from those providing trans-acting viral proteins. Usually three plasmids are produced (fig.1.8): a packaging construct, expressing structural and enzymatic viral proteins provided by ORF gag and pol; a vector construct encoding the transgene and the cis-acting sequences necessary for viral replication, retrotranscription, integration and encapsidation. In fact, vector construct is the unique plasmid containing ψ sequence and therefore the only one encapsidated in vector particles. The third plasmid is the env construct encoding surface GPs; it can express either homologous or heterologous viral GPs.
23 For vector production these three plasmids are used to transfect human embryonic kidney cells (293T) in which the constructs are transcribed and translated leading to the assembly of vector particles that bear vector construct in place of wild-type viral genome. These particles are then used for transduction of specific target cells in which gene delivery must be carried out. In fact, following binding between cellular and viral receptors RNA is released in host cell cytoplasm, reverse transcribed, integrated and through cell machinery; it expresses the protein codified by the transgene (fig.1.9).
One of the main advantages of these vectors is that providing required viral sequences in separate plasmids reduces risks of recombination and wild type virus formation. From this idea the third generation lentiviral vector further improved their safety by becoming self-inactivating (SIN). Following transduction the vector loses some cis-acting sequences so that, once the gene delivery has been carried out in target cells, it cannot replicate anymore.
For SIN vector production U3 region at 3’LTR is deleted of a portion containing TATA box and Sp1 and NF-kB transcription factors binding sites (Pistello et al., 2007). Since this deletion is transferred to the 5’ LTR during reverse transcription, in transduced cells, both LTRs are inactivated and the only region transcribed is the expression cassette in which an internal promoter drives the expression of the transgene. This fact, besides abolishing the danger connected with viral replication, reduces also the risk related to insertional mutagenesis, a phenomenon occurring during integration. In fact, when virus integrates into host cell genome it may break a gene coding sequence stopping its expression. Moreover if the insertion takes place upstream a normally “switch off” gene, LTRs acting as promoters can trigger gene transcription and this might lead to unchecked cell growth. The latter problem can be reduced using SIN vectors whose LTRs are inactive. Many HIV-1 vectors have been developed along these rules and are capable to transduce a broad spectrum of cells in vitro and in vivo (Ricks et al., 2008) (Chang & He, 2001) (Kordower et al., 2000) (Consiglio et al., 2001),
Anyway, as HIV-1 is a very dangerous pathogen that causes a lethal disease in humans its employ in clinical trial tends still to be avoided. To exploit lentiviruses features without the using human pathogens, numerous lentiviral vectors have been produced starting from viruses that cannot infect human such as simian immunodeficiency virus (SIV) or feline immunodeficiency virus (FIV).
24 Figure 1.9: Lentiviral vector’ production and mechanism of action production mechanism.
25
1.10 FIV AND FIV VECTORS
FIV is an enveloped virus containing two GPs: surface (SU) and transmembrane (TM) glycoprotein. An internal capsid packages 2 copies of RNA genome sized 9,4 kb (fig. 1.10) containing the 2 viral promoter 5’and 3’LTRs, gag, pol and env ORF and three other shorter ORFs encoding for regulatory proteins Orf-A, Vif and Rev.
FIV is very similar to HIV-1 as regards morphology and organization of proviral genome. It infects felines causing pathology comparable to human AIDS.
Nevertheless humans have been widely exposed to FIV via bites or scratches, which are the principal and very efficient natural mode of inter-feline transmission, and which are known to frequently transmit other pathogens, seroconversion has never been observed (Willett et al., 2003). This restriction is due to little feline LTRs activity, and low envelope tropism in human cells. Although there are no substantial grounds to doubt that HIV-1 might be adapted to clinical use safely, the employ of a virus that is not pathogen for humans represents a further safety for human gene therapy.
Figure 1.10: FIV particle and proviral genome.
To adapt a FIV model to human cells is essential to modify viral GPs and promoters. To this purpose, in the first FIV vector system constructed from FIV34TF10 (Talbott et al., 1989) in 1998, U3 region of 5’LTR was replaced with a stronger and constitutively expressed promoter derived from human cytomegalovirus (CMVp).
This modification allowed higher vector particles production from human cells and transduction of human quiescent cells (Poeschla et al., 1998). Further optimizations led to development of second and third generation FIV vectors that are SIN and in which most of not essential viral proteins were deleted (Curran et al., 2000) (Pistello et
Orf A
LTR
gag
pol
vif rev
26 al., 2007). As for HIV-1, FIV vectors are produced by transfection of three components: vector, packaging and env construct.
Vector construct: this plasmid contains the transgene that must be vehiculated into target cells. The exogenous DNA is expressed under a specific internal promoter control, usually CMVp or phosphoglycerate kinase (PGK) promoter. An optimal vector construct should exclude all viral sequences except for the encapsidation signal and crucial elements for retrotranscription and integration. These elements are included in R and U5 region of 5’ LTR, mayor splice donor and the first 120 nt of gag region (Pistello et al., 2007). Another important sequence is the rev responsive element (RRE) region that belongs to Rev/RRE system essential for efficient nuclear export of unspliced vector construct RNA. Most other elements derive from heterologous viruses such as the CMVp, previously described and the woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) able to increase transgene mRNA expression when inserted downstream the expression cassette (Donello et al., 1998) (Pistello et al., 2007).
Packaging construct: this plasmid provides in trans capsid (p25), matrix (p15) and nucleocapsid protein (p8) encoded by ORF gag and reverse transcriptase (RT) protease (PR) integrase (IN) and dUTPase (DU) expressed by ORF pol all necessary for virus life cycle. It also encoded the protein Rev to rescue the RRE-containing vector RNA. To facilitate expression in 293T transfected cells 5’LTR is completely replaced with CMVp whereas 3’LTRis substituted with bovine growth hormone polyadenilation signal (BGH-A). Env ORF and ψ are both deleted.
Env construct: this plasmid is used to pseudotype the vector. As FIV GPs determine FIV restricted feline tropism, they are usually replaced with heterologous GPs. Vescicular stomatitis virus glycoprotein G (VSV-G) is one of the most commonly used GP for this purpose. This protein uses a ubiquitous membrane phospholipid as cellular receptor allowing to widely broadening cell tropism.
Recent studies showed FIV vector capability of transducing human, murine cell lines and primary cells also using a heterologous glycoprotein derived from RD114 feline endogenous virus (RD114/TR) (Pistello et al., 2007). Other gammaretroviruses GPs proved efficient in transducing CD34+ hematopoietic progenitor cells, T lymphocytes
27 and mesenchymal stem cells. Alternative GPs derived from rabies virus, mokola virus were also tested to reach neuronal cells (Cronin et al., 2005).
1.11 FIV VECTORS FOR IN VIVO USE.
Lentiviral vectors in vivo experiments can be carried out using a direct or indirect way. In the in vivo direct method lentiviral particles, previously produced by 293T cells co-transfection, are straight injected into the patient or experiment animal. In the ex vivo indirect way target cells are collected from the patient or experimental animal, transduced with the vector and the re-injected. In most cases the type of pathology to treat and the accessibility of the body region are the main factors to consider in the strategy choice. FIV vector are successfully been applied in vivo for transduction of different cell types. Due to lentiviruses capability of infecting non-dividing cells, most FIV vectors have been tested for in vivo transduction of quiescent cells such as neurons, lymphocytes, hepatocytes and stem cells. FIV vectors proved highly efficiently in transducing immune cells and central neuron system (CNS) cells such as white matter and retina when injected intraperitoneally (Kyrkanides et al., 2003). Ocular gene therapy, in fact, could offer novel approaches to treating eye diseases characterized by retinal neovascularization (e.g., diabetic retinopathy, age related macular degeneration, retinopathy of prematurity), or chronic retinal degeneration (e.g., retinitis pigmentosa, chorioideremia, atrophia gyrata, Best’s disease, Stargardt’s disease). Several different viral vector systems have been proposed, including lentiviral and Ad vectors. Through a FIV vector, a long-term transduction of retinal pigment epithelium (RPE) was carried out following subretinal injection in guinea pig. Ad vectors proved also able of transducing RPE but at lower efficiency compared to FIV vectors. Moreover Ad vectors transduction resulted in higher cellular infiltrate, and caused sensible disruption of retinal architecture probably related to Ad pre-existing immunity (Loewen et al., 2004). Instead FIV vectors proved able also of transducing white matter important in creating interaction between different cerebral regions and playing a key role in development, aging, and many neurologic and psychiatric disorders across the life span (Filley, 2005), and also implicated in some brain tumors and multiple sclerosis.
Gene therapy could be easily applied to pathologies originated by a single gene mutation such as cystic fibrosis caused by a mutation in the chloride ion channel
28 gene known as cystic fibrosis transmembrane conductance regulator (CFTR). Ad vectors are potentially good candidates as they naturally infect respiratory epithelium, but they cause a high immune response and the transgene expression slowly decreases in time. FIV vectors expressing CFTR corrected deficient Cl- transport in human respiratory epithelium cells, but in vivo they could only transduce 14% of rabbit respiratory epithelium (Wang et al., 2002). In fact, the main limitation for in vivo gene therapy is to transduce a great number of target cells efficiently. A way improve this aspect is to find a suitable heterologous GP. In the case of respiratory epithelium different GPs were tested for ability to bind folate receptor alpha, highly expressed in respiratory epithelium such as GP from the filoviruses Marburg virus and Ebola virus (Sinn et al., 2003) or, more recently, GP64 from Autographa californica ulticapsid nucleopolyhedrovirus that proved able to mediate persistent gene transfer to the respiratory epithelia of mice (Sinn et al., 2005). FIV vectors were also tested for treating β−talassemia, liver pathologies, and rheumatoid arthritis (May et al., 2002) (Condiotti et al., 2004) (Lin et al., 2004).Viral vectors can be also attractive tools to develop innovative vaccination strategies against pathogens or cancer. In fact they could be used to vehiculate antigen encoding transgenes to develop a strong humoral and cellular specific immune response. In this context, the main target cells are dendritic cells (DCs) which are the most important antigen presenting cells (APC) and lymphocytes. Among different virus families tested for transduction of primary immune cells, lentiviruses proved the most efficient because of their innate capability of infecting quiescient cells. Several studies have been carried out to evaluate lentiviral vector capability of transducing primary immune cells demonstrating its functionality both through in vivo and ex vivo techniques. Indeed a lentiviral vector injected in mice footpad led to DCs transductions in the draining lymph node and in the spleen. (Esslinger et al., 2003) Moreover murine DCs ex vivo transduced with a lentiviral vector expressing murine tyrosinase-related protein 2 (mTRP-2) transgene (a clinically relevant melanoma-associated antigen) and re-injected in mice induced protective immunity a complete protection of mice from further melanoma cell challenge (Metharom et al., 2005). In addition a recently developed FIV vector demonstrated good capability of ex vivo transducing both primary DCs and lymphocytes when pseudotyped with VSV-G or RD114/TR (Pistello et al., 2007) laying the basis for the development of innovative and more specific vaccination strategies.