1
Rational Design, Synthesis and
Pharmacological Evaluation of New
Cannabinoid CB
2
Receptor Ligands
Director of PhD School: Candidate:
Adriano Martinelli Valentina Lucchesi
A thesis submitted in fulfillment of the requirements for the degree of Doctor of Clinic Physiopathology and Science of Drugs, University of Pisa, 2013.
Table of Contents
Abstract………..………..…i-ii
List of Figures…………..……….iii
List of Tables.………....iv
General Introduction………..….………….….…………...….……….1
Endocannabinoid system (ECS)………….………….….…………...2
Endocannabinoids ……… ………...2
Cannabinoid Recerptors………...5
Physiological actions of ECS………...……...…………..……..9
Licensed Medicines that target cannabinoid receptor………….……….9
Therapeutic potential of cannabinoid………...…..13
Activating Cannabinoid CB2 Receptor……….13
Role of CB2 in Neuroinflammation………….………14
Cannabinoid CB2 receptor ligands ………..…………22
Introduction of Experimental Section……….….………..…….28
Identification of 1,8-naphthyridin-2(1H)-on-3-carboxamide derivatives as new potent and selective ligands for cannabinoid CB2 receptor: synthesis, pharmacological evaluation and docking studies..…...….35
Development of 1,8-naphthyridin-2(1H)-one derivatives for PET imaging of cannabinoid CB2 receptor……….………40
Rational design, synthesis and pharmacological evaluation of new cannabinoid receptor ligands: an investigation of the 1,8-naphthyridin-2(1H)-one scaffold……….………..…………...…65
Experimental Section…………..……….……….77
Abstract
Since the discovery of the cannabinoid receptors and their endogenous ligands, numerous studies implicate the endocannabinoid system in several physiological and pathological processes including cancer, appetite, fertility, memory, neuropathic and inflammatory pain, obesity, neurodegenerative diseases etc. At the present time, CB2 receptor has gained attention as potential target for its
immunoregulatory effects. Recent advances suggest a role for the CB2 receptors
within the nervous system, particularly in inflammatory conditions such as neurodegenerative diseases (Parkinson’s disease, Alzheimer’s disease, Huntington’s disease,multiple sclerosis, etc.), given CB2 receptor upregulated in
the brain under these conditions and disease states. Thorough pharmacological characterization of the CB2 receptor is necessary for our understanding of the
specific role this target plays in both the periphery and the central nervous system.
Therefore, this Phd thesis is focused on the research for CB2 selective ligands.
Several compounds, based on the previously reported 1,8-naphthyridin-2(1H)-one derivatives (Manera et al., 2009), were synthesized, screened for binding and activity at human cannabinoid receptors. Generally, these compounds exhibited a remarkable CB2 affinity, with a Ki value in the nanomolar range, and
high selectivity with respect to CB1 receptor. Some agonists and
antagonists/inverse agonists were identified within this series. Interestingly data obtained demonstrated to act as agonists or inverse agonists/antagonists in functional activity assays, depending on the presence of the substituents at position C-6 of the naphthyridine scaffold. Docking studies confirmed that the introduction of substituents in this position determined a functionality switch from agonist to antagonist. Moreover one of these compounds has been chose for development of new candidate for PET imaging of CB2 receptor.
Finally,using our previously described series of 1,8-naphthyridin-2(1H)- on-3-carboxamides as a lead class, several nitrogen heterocyclic derivatives, characterized by different central cores C-F, were synthesized and tested for their affinity toward the human CB1 and CB2 cannabinoid receptors. The
obtained results suggest that the new series of quinolin-2(1H)-on-3-carboxamides, 4-hydroxy-2-oxo-1,2-dihydro-1,8-naphthyridine-3-carboxamides and 1,2-dihydro-2-oxopyridine-3-carboxamides represent novel scaffolds very suitable for the development of promising CB2 ligands.
Furthermore some of these compounds exerted anti-proliferative effects on LNCaP cell line.
List of Figures
Figure 1. Chemical structures of some pharmacologically active plant cannabinoids………..1
Figure 2. Chemical structures of biologically active eCBs and of the eCB-like compounds....3
Figure 3. Endocannabinoid synthesis and degradation pathways………..4
Figure 4. CB1 and CB2 receptors structures………...6
Figure 5. The involvement of ECS in some pathophysiological conditions...………...9
Figure 6. Inflammation, neurodegeneration, and endocannabinoid system (ECS) in chronic central nervous system (CNS) diseases………..………..…20
Figure 7. Most known cannabinoid CB2 receptor ligands…………..……….22
Figure 8. Photographs of a representative vehicle-treated and A1-treated tumor………...30
Figure 9. General structures of the naphthyridin-4(1H)-on-3-carboxamide (A) and 1,8-naphthyridin-2(1H)-on-3-carboxamide (B) derivatives and structures of compound A2 and B1………..31
Figure 10. 3D structure and solvent-excluded molecular surface of compound A2 (A), B1 (B), and their superimposition (C)…....………....32
Figure 11. Some CB2 radioligands reported in literature………..………..….41
Figure 12.The Microfluidic Flow-Chemistry System, NanoTek®……….46
Figure 13. Signaling Pathways Mediated by CB2 Agonist in U2OS cells co-expressing CB2 and βarr2-GFP………...54
Figure 14. Concentration response curves depicting βarr2-GFP recruitment following treatment with CB2 agonists………..55
Figure 15.WIN 55212-2 mediated βarr2-GFP recruitments is blocked by CB2 antagonists…………55
Figure 16. Representative images, captured with confocal microscopy at 40x magnification depicting βarr2-GFP recruitment pattern are illustrated……….56
Figure17. CB2 Agonists inhibit cAMP formation……….…….…..57
Figure 18. Treatment with the CB2 antagonist B27 inhibits the response to WIN55,212-2…57 Figure 19. B26-cis bound in the CB2 R receptor………..………..62
Figure 20. B12-cis bound in the CB2 R* receptor………...………63
Figure 21. B26-cisbound in the CB2 R receptor………..64
Figure 22. Pharmacophoric superimposition of the analyzed scaffolds……….66
Figure 23. Dose-response curves of E3 and C10 tested in CHO-hCB2 cell membranes…...74
Figure 24. Concentration response curves depicting βarr2-GFP recruitment following treatment with CB2 agonists, E3 and WIN 55212,2……….…75
Figure 25. Effect of C10 and E3 on LNCaP cell viability………..76
Figure 26. Effect of JWH-133 on LNCaP cell viability………...………..76 iii
List of Tables
Table 1. Radioligand Binding Data of Compounds B1-B12………...…………...33 Table 2. PET radionuclide………...……….………42 Table 3. Radioligand binding data of 1,8-Naphthyridin-2(1H)-on-3-carboxamide derivatives B13-B25 and B36-B43………..………..………48-49 Table 4. Radioligand binding data of 6-substitued-1,8-naphthyridin-2(1H)-on-3-carboxamide derivatives B26-B35………..………..……51-52 Table 5. Affinities and Potencies of CB2 ligands obtained from Binding, Second Messenger
and Receptor β-Arrestin Interaction Assays………58-59 Table 6. Radioligand binding data of compounds C-F………...…70-71
1
General Introduction
Preparations from Cannabis sativa have been used for millennia, and yet the chemical and biological bases of their pharmacological effects are still not fully understood. Cannabis plants produce a unique family of terpenophenolic compounds, which produce the "high" one experiences from smoking marijuana. The two cannabinoids usually produced in greatest abundance are cannabidiol (CBD) and/or Δ9- tetrahydrocannabinol (THC), but only THC is psychoactive. These compounds were identified in the 1960s (Mechoulam and Shvo, 1963; Gaoni and Mechoulam, 1964) and, later, of more than 60 other plant cannabinoids were isolated and characterized (Figure 1). This, followed by the discovery of the body’s own cannabinoid system with specific receptors and endogenous ligands, marked the beginning of intensive research into the function of the endocannabinoid system and the clinical relevance of cannabis-based medications.
Figure 1. Chemical structures of some pharmacologically active plant cannabinoids (or phytocannabinoids).
2
The Endoannabinoid System (ECS)
The endogenous cannabinoid system is a complex biochemical system that appeared early in evolution and which has important regulatory functions throughout the body. The endocannabinoid system includes: (I) cannabinoid CB1 and CB2 receptors, (II) exogenous and endogenous cannabinoids and (III)
enzymes responsible for the production, transport, and degradation of these ligands (Scotter et al., 2010).
Cannabinoids (CBs) are a family of molecules that includes phytocannabinoids, synthetic CB-mimetic compounds (synthetic cannabinoids), and endogenous ligands of CB receptor (endocannabinoids).
Endocannabinoids
Endocannabinoids (eCBs) are lipid mediators, derivatives of integral components of the cellular membranes that include amides, esters, and ethers of long chain polyunsaturated fatty acids; they mimic the action of ∆9-tetrahydrocannabinol (THC) in different biological processes (Battista et al., 2012).
At the present moment, the most bioactive and best characterized eCBs are anandamide (arachidonylethanolamide; AEA) and 2-arachidonoylglycerol (2-AG), yet the eCBs family includes also virodhamine, noladin ether, and N-arachidonoyl dopamine (NADA), and other cognate compounds such as palmitoylethanolamide (PEA) and oleoylethanolamide (OEA) (Figure 2).
3
Figure 2. Chemical structures of biologically active eCBs and of the eCB-like compounds.
eCBs are released “on demand” from membrane phospholipid precursors. Different pathways are involved in the synthesis and release of N-acylethanolamines (NAE)- AEA, PEA , OEA- and of 2-AG (Figure 3) (Alhouayek and Muccioli, 2012). The NAE biosynthetic enzyme, N-acyltransferase, catalyses the transfer of arachidonic acid from phosphatidylcholine to phosphatidylethanolamine, synthesizing the precursor N-arachidonoyl-phosphatidylethanolamine (NAPE) of N-acylethanolamines. A specific phospholipase D (NAPE-PLD) catalyses the release of AEA, PEA and OEA (Cadas et al., 1996). An alternative parallel pathway involves hydrolysis of NAPE by phospholipase C (PLC), followed by dephosphorylation through a phosphatase (Liu et al., 2006). 2-AG synthesis is closely associated with the metabolism of triacylglycerol, driven by the receptor dependent activation of phosphatidylinositol-specific PLC and finally diacylglycerol lipase (DAGL) (Bisogno, 2005).
4
Figure 3. Endocannabinoid synthesis and degradation pathways (Alhouayek and Muccioli, 2012).
Endocannabinoid levels are maintained by a two step process that includes transport into cells and subsequent intracellular hydrolysis.
The cellular uptake from the extracellular to the intra cellular space is accelerated by a rapid and selective carrier system that is likely to take up both AEA and 2-AG (Beltramo et al., 1997; Hillard et al., 1997). Until now, several lipid carrier proteins have been cloned (Amburad et al., 1999) and there is wide experimental evidence to support the concept that AEA transport across membranes is protein-mediated, but the molecular identity of this carrier is still lacking.
Endocannabinoid signaling is terminated by several catabolic enzymes: fatty acid amide hydrolase (FAAH) for NAE, monoacylglycerol lipase (MAGL) and α/β-hydrolase domain 6 (ABHD6) for 2-AG (Ueda, 2002; Dihn et al., 2002), even if evidence exists for the involvement of FAAH in the control of 2-AG levels (Di Marzo and Maccarone, 2008). Moreover, other enzymes showing
5
“amidase signature” such as FAAH-2 and the N-acylethanolamine-hydrolyzing acid amidase (NAAA), which belongs to the choloylglycine hydrolase family, might bind with low affinity and hydrolyse AEA to release arachidonic acid and ethanolamine (Battista et al., 2012). Also cyclooxygenase-2 (COX-2), different lipoxygenase (LOX) isozymes and cytochrome P450 are able to accept AEA and 2-AG as a substrate, leading to the biosynthesis of prostaglandin ethanolamides and glycerylesters, hydroxy-anandamides, and hydroxyl- eicosatetraenoic-glycerols, respectively.
Cannabinoid Receptors
Howlett confirmed the existence of cannabinoid receptors showing that cannabinoids decreased cAMP in neuroblastoma cell cultures (Howlett, 1984). This date suggested the mediation by a Gi/o-coupled receptor (Howlett, 1985;
Howlett and Fleming, 1984). Even immunohistochemical and radioligand binding methods allowed the determination and characterization of a cannabinoid receptor from the brain (Devane et al.,1988). Until now, two cannabinoid receptors (the type 1 (CB1) and type 2 (CB2) receptors), have been
described with regard to their primary structure, ligand-binding properties, and signal transduction systems (Howlett et al., 2002; Pertwee et al., 1995). The CB1
and CB2 receptors are members of the G protein-coupled receptor (GPCR)
family and thread through cell membranes seven times (heptahelical receptors). They are characterized by a N-terminal extracellular domain that possesses glycosylation sites, a C-terminal intracellular domain coupled to a G protein complex, and 7 hydrophobic transmembrane segments connected by alternating extracellular and intracellular loops.
The cannabinoid receptors are expressed in many species, including human, monkey, pig, dog, rat and mouse, but not insects. Initially, it was believed that the CB1 receptor was localized predominantly in the brain (central receptor for
6
cannabinoids), whereas the CB2 receptor in peripheral cells and tissues derived
from the immune system (peripheral receptor for cannabinoids) (Svíženská et al., 2008). However, the CB1 receptorhas recently been found also in a number
of peripheral tissues, such as the cardiovascular and reproductive systems as well as the gastrointestinal tract. On the other hand, the CB2 receptor was
recently detected also in the central nervous system(CNS), e.g., in the microglial cells as well as the neurons.
The CB1 receptor cDNA was isolated first from a rat cerebral cortex library
using an oligonucleotide probe derived from a member of G protein-coupled receptors (Matsuda et al., 1990). The gene locus for the human CB1 receptor has
been localized in chromosome 6 to position 6q14–q15 (Caenazzo et al.,1991). A second gene encoding the human CB2 receptor was cloned in 1993 and located
in chromosome 1p36 (Raitio et al., 2005). The human CB2 receptors has 44%
amino acid sequence identity with CB1 receptor throughout the total protein
(Munro et al., 1993).
Figure 4. CB1 and CB2 receptors structures.
Both CB1 and CB2 receptors are coupled with Gi or Go protein, negatively to
7
(Svíženská et al., 2008). CB1 coupling to the G protein signal transduction
pathways in presynaptic nerve terminals transduces the cannabinoid stimulation of MAP-kinase and inhibition of adenylyl cyclase, thus attenuating the production of cAMP. CB1 are also coupled to ion channels through Gi/o
proteins, positively to A-type and inwardly rectifying potassium channels, and negatively to N-type and P/Q-type calcium channels and to D-type potassium channels. Due to the decrease of cAMP accumulation, cAMP-dependent protein kinase (PKA) is inhibited by CB1 activation. In the absence of cannabinoids,
PKA phosphorylates the potassium channel protein, thereby exerting decreased outward potassium current. In the presence of cannabinoids, however, the phosphorylation of the channel by PKA is reduced, which leads to an enhanced outward potassium current. Based on these findings, it has been suggested that cannabinoids play a role in regulating neurotransmitter releases. Inhibition of presynaptic calcium channels by cannabinoids likely reduces neurotransmitter release from CB1-expressing presynaptic terminals. The CB2 receptor is also
coupled to Gi/o proteins and thereby negatively coupled to adenylyl cyclase and the cAMP pathway in various types of cells, and it stimulates mitogen-activated protein kinase (MAPK) cascades. Inwardly rectifying potassium channels can also serve as a signaling mechanism for the CB2. CB2 receptors are located
principally in immune cells, among them leucocytes and those of the spleen and tonsils. One of the functions of CB receptors in the immune system is modulation of cytokine release. Activation of B- and T-cell CB2 receptors by
cannabinoids leads to inhibition of adenylyl cyclase in these cells and to a reduced response to immune challenge.
eCBs act principally through CB1 and CB2 receptor but recently, it has been
highlighted the ability of some CB and non-CB ligands to bind also other receptors like the serotonin type 3 receptor, α7-nicotinic acetylcholine receptor and the transient receptor potential vanilloid type 1 receptor (TRPV1), a non selective cationic channel (Zygmunt et al., 1999). Cannabinoid effects independent of any kind of known receptor have also been reported, what
8
suggests a direct diffusion through the lipid bilayer of cell membranes or the presence of other unidentified cannabinoid receptor subtypes (Mackie and Stella, 2006). In fact, also nuclear receptors like the peroxisome proliferator-activated receptors (PPARs) have been added to the list of eCBs targets, activated under physiological and pathological conditions (Pistis and Melis, 2010). As a consequence, PPARs activation affects several physiological and pathological processes, such as lipid metabolism, energy balance, and feeding behavior, neuroprotection, epilepsy, circadian rhythms, inflammation, addiction, and cognitive functions (Pistis and Melis, 2010).Finally, the orphan GPR55 and GRP119 receptors have recently attracted much attention as another member of the cannabinoid receptor family (Battista et al., 2012). In particular the activation of GPR55, the purported “CB3 ” cannabinoid receptor, has been
linked to (1) intracellular Ca2+ increase; (2) activation of the small GTPase proteins Rho A, Rac, and Cdc42 and (3) ERK phosphorylation.
Furthermore, eCBs exhibit different binding properties at CB1 and CB2 receptors
and at the others putative target. AEA and 2-AG bind CB1 and CB2 receptors
and PPARs, but they showed different intrinsic activity at these receptor (Mackie and Stella, 2006). For instance, PEA and OEA, differing from AEA by their acyl chain, do not bind to cannabinoid receptors but to the nuclear receptors PPAR and to G protein-coupled receptors such as GPR119 and GPR55. The receptor channel TRPV1 is activated by AEA and N-arachidonoyl-dopamine. Addionally, AEA is also implicated in other signaling pathways and, in particular it has been observed that muscarinic and glutamate receptors have allosteric sites for AEA binding (Lanzafame et al., 2004). Finally, eCBs also reduce the release of biogenic amines noradrenaline and serotonin, neuropeptide CCk-8, acetylcholine and GABA (Manzanares et al., 1999; Ghozland et al., 2002). This highly complex network of interactions is reflected in the multifaceted modulatory effects of eCBs on the regulation of brain and behavioral functions (López-Moreno et al., 2008).
9
Physiological actions of ECS
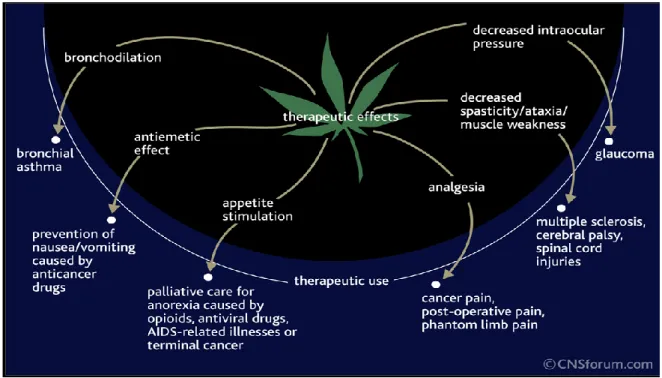
The ubiquitous presence of the endogenous cannabinoid system correlates with its role as a modulator of multiple physiological processes, including appetite, analgesia, fertility, immune functions, memory, intraocular pressure and bronchodilation (Ligresti et al., 2009; Maccarrone et al., 2010) (Figure 5).
Figure 5. The involvement of ECS in some pathophysiological conditions.
In the last 15 years, it has become clear that a dysregulation of ECS is connected to pathological conditions, and thus its modulation through inhibition of metabolic pathways and/or agonism or antagonism of its receptors are pharmacological tools for the study of the ECS for research and intervention in multiple areas of human health (Battista et al., 2012).
Licensed Medicines that target cannabinoid receptor
Cannabinoids are best known for their effects on CNS functions. They produce euphoria, alterations in cognition and analgesia, have anticonvulsant properties
10
and affect temperature regulation, sleep and appetite (Pertwee, 2012). Based on these knowledge plant-derived cannabinoids, synthetic cannabinoids, and eCBs have been tested as novel therapeutics in a wide range of in vivo and vitro model and clinical trials. At the present, only three medicines that activate cannabinoid receptors are now in the clinic: Cesamet®, Marinol® and Sativex®. These medicines are used mainly for:
Anti-emetic effects: the therapeutic applications of cannabis plant have been
studied for several centuries, including the attenuation of nausea and vomiting (Mechoulam, 2005). Cancer chemotherapy can induce nausea and vomiting which vary in intensity, but which can sometimes be severe and prolonged. Ineffective treatment of these side-effects prompted oncologists to investigate the anti-emetic properties of cannabinoids in the late 1970s and early 1980s, before the discovery of the 5-HT3 antagonists (Parker, 2011). The first
cannabinoid agonist, nabilone (Cesamet®), which is a synthetic analogue of Δ9- THC was specifically licensed in 1981 for the suppression of nausea and vomiting produced by chemotherapy. Later, also dronabinol (a synthetic Δ9-THC), entered the clinic as Marinol® as an antiemetic. Moreover, several clinical trials compared the effectiveness of Δ9-THC with placebo or other anti-emetic drugs. Comparisons of oral Δ9-THC with existing antianti-emetic agents generally indicated that Δ9-THC was at least as effective as the dopamine antagonists, such as prochlorperazine.
Appetite stimulation: severe loss of appetite and a progressive weight loss are
frequently experienced by patients suffering from an advanced stages of cancer or HIV infection. Moreover, in the case of AIDS, cachexia (extreme weight loss) may be accompanied by chronic diarrhea and weakness (Iversen, 2000). Two controlled studies have demonstrated that oral THC stimulates appetite and helps retard chronic weight loss in patients with cancers and AIDS. It also observed that the treatment with dronabinol tended to stabilize weight, while patients on placebo continued to lose weight. Moreover, this effect persisted in
11
the patients who continued to receive dronabinol after the end of the study (Beal et al., 1995). Marinol® was licensed in 1992 as an appetite stimulant.
Obesity: the CB1 receptor appears to regulate the activity of mesolimbic
dopamine neurons, thereby possibly modulating hedonistic or reward behaviors mediated by dopamine and to interact with neuropeptides such as the melanocortins and gut peptides such as ghrelin in regulating food intake (Kishore et al., 2006). Moreover, several reports documented that the selective pharmacologic antagonism the CB1 receptor improves lipid abnormalities
associated with obesity (Matias and Di Marzo, 2007). Following the good outcome obtained in various clinical trials, the best known CB1 blocker
SR141617A, also called rimonabant (and commercially known as Acomplia®) had been approved in Europe in 2006 for the treatment of obesity; while it did not receive approval in the USA or Canada due to safety concerns. Despite its positive effects on body weight, the European Medicines Agency (EMEA) in October 2008 recommended the suspension of the sale of rimonabant due to safety concerns about the adverse effects in patients taking it, particularly an increased incidence of depression and suicidality (Van Gaal et al., 2005). Rimonabant does of course remain an extremely valuable experimental tool. However, it is likely that most pharmaceutical companies will be deterred, at least for the time being, from developing a drug that displays rimonabant-like CB1 receptor inverse agonist/antagonist activity for the management of any
disorders (Pertwee, 2009). So, there is an urgent need for a new strategy for blocking CB1 receptors that shares the generally acknowledged effectiveness of
rimonabant against obesity, type-2 diabetes and associated cardiometabolic risk factors but not its apparent ability to produce signs of anxiety and marked depression/suicidal ideation in some patients. Possible solutions to this problem could be the development of a neutral antagonist because it lacks the CB1
inverse agonist activity of rimonabant, developing a peripherally restricted drug that selectively blocks CB1 receptors expressed outside the brain or an allosteric
12
of the endocannabinoids, or applying an adjunctive strategy that exploits synergism between a low dose of a CB1 receptor antagonist and some other type
of anti-obesity agent.
Neuropathic pain: it is a debilitating form of treatment-resistant chronic pain
caused by damage to the nervous system. Neuropathic pain may result from peripheral nerve injury, toxic insults, and disease states such as diabetes, human immunodeficiency virus (HIV), multiple sclerosis (MS), and herpes zoster infection. Neuropathic pain remains a significant clinical problem because it responds poorly to available therapies. Cannabinoids have been evaluated in clinical studies for the suppression of acute, postoperative and neuropathic pain (Rahn and Hohmann, 2009). Based upon of the literature, cannabinoids exhibit their greatest efficacy when employed for the management of neuropathic pain. Sativex®, a mixture of THC and the non-psychoactive plant cannabinoid, cannabidiol, was licensed in Canada in 2005 for the symptomatic relief of neuropathic pain in multiple sclerosis and as an adjunctive analgesic treatment for adult patients with advanced cancer.
Spasticity: it remains a prevalent symptom in multiple sclerosis, with a
significant associated disability and quality of life impairment. A significant improvement in therapy aimed at reducing multiple sclerosis relapses and modifying its course has been achieved in recent years (Oreja-Guevara, 2012). Both general and specific traditional treatments have, however, major limitations. Thus, its use in real practice is lower than expected. Cannabinoids provide a new way for therapy. In 2010, Sativex® was licensed in the UK and Canada for the treatment of spasticity due to multiple sclerosis and has more recently become an approved medicine in several other countries. Sativex®, administered through an oromucosal route, causes a specific effect on CB1 and
CB2 receptors, with traditional psychotropic cannabis actions being minimized.
Randomized, placebo-controlled trials, as well as longer-term open-label extensions, have shown a clear-cut efficacy to reduce spasticity and their associated symptoms in those patients refractory to other therapies, with a good
13
tolerability/safety profile. No tolerance, abuse or addictive issues have been found.
Therapeutic potential of cannabinoids
Cannabinoids also have a number of potential therapeutic targets (reviewed in Pertwee and Thomas, 2009). These include the:
inhibition of angiogenesis and growth of malignant tumours;
management of neurodegenerative diseases (multiple sclerosis, spinal cord injury, Alzheimer's disease and amyotrophic lateral sclerosis);
treatment of some gastrointestinal disorders;
management of atherosclerosis and of certain other cardiovascular disorders;
relief from tics and behavioural problems experienced by patients with Tourette's syndrome;
management of anxiety disorders, attention-deficit hyperactivity disorder, depression and brain repair;
management of tardive dyskinesia induced in psychiatric patients by neuroleptic drugs;
management of glaucoma, cough and cholestatic pruritus.
Activating Cannabinoid CB
2Receptor
Cannabinoid receptor ligands can induce adverse effects in patients. The most frequently reported adverse reactions include: dizziness/light-headedness, dry mouth, tiredness/fatigue, muscle weakness, myalgia (muscle pain) and palpitations (Pertwee, 2009). Disorientation, feeling of drunkenness, ‘high sensation’, mental clouding and/or altered time perception, impairment of memory or ability to concentrate, tremor, balance impairment or lack of coordination, nausea/feeling sick, hypotension, blurred vision, constipation or
14
diarrhoea, confusion, dysphoria/depression, disorientation, paranoia and hallucinations are minor side effects reported (Pertwee, 2009; Pertwee and Thomas, 2009). These adverse effects result from CB1 receptor rather than from
CB2 receptor activation. For this reason, considerable attention is currently being
directed at the possibility of developing medicines from compounds that can activate CB2 receptors at doses that induce little or no CB1 receptor activation
(Pertwee, 2012). Moreover CB2-selective agonists have a number of important
potential therapeutic applications. These include the relief of various kinds of pain and the treatment of pruritus, of certain types of cancer, of cough and of some immunological, inflammatory, cardiovascular, hepatic, renal and bone disorders. However, CB2 receptor activation has gained more attention in
neuroinflammatory states and neurodegenerative disease.
Role of CB
2in Neuroinflammation
Inflammation is defined as "the response of living vascularized tissues to harmful agents”. It involves complex biological processes that act as a protective mechanism to remove the injurious stimuli, whereas it can lead to tissue damage if self-perpetuating. In the central nervous system, the activation of resident immune cells and the influx of immunocytes from peripheral non-neuronal sites after the breakdown of the blood–brain barrier (BBB) plays a critical role in neuropathological processes related to chronic neuroinflammation (Rossi et al., 2010). Both astrocytes and microglia are the most involved cells in the neuroinflammation, while neurons are assumed to play a largely passive role, being only the victims of immune responses. Microglia are cells that reside within the parenchyma of the nervous system, that share many if not all the properties of macrophages in other tissues, but that in their nonactivated or resting state have a characteristic “ramified” morphology not seen in resident macrophages of other organ systems (Rock et al., 2004). In response to CNS injury, microglia cells quickly convert to on “active” state during which they
15
change to on amoeboid shape. Microglia act during early stages of neuroinflammation and once activated produce proinflammatory factors including the interleukin (IL), and tumor necrosis factor α (TNFα), nitric oxide, oxygen radical, glutamate, proteases. These proinflammatory mediators contribute to the pathogenesis of neurologic disorders. In fact, they have cytotoxic effect on neurons and they can activate astrocytes leading to a further induction of the expression of inflammatory factors and to breakdown of the BBB (Rossi et al., 2010). Moreover, immune attack of the CNS induces damage to the oligodendrocytes that form myelin but also to the neurons themselves creating a microenvironment with many demyelinated axons and neuroinflammatory effectors and sustaining neurodegeneration.
As mentioned, the CB2 receptor is mainly expressed in immune cells, such as
macrophages (Parolaro, 1999), oligodendroglial cells (Cabral and Marciano-Cabral, 2005), and lymphocytes B and T (Lee et al., 2001; Börner et al., 2009). In presence of inflammatory stimuli, the activation of immune cells modulates the expression of CB1 and CB2, a fact that has been linked to the immune
regulatory effects of cannabinoids (Patel et al., 2010). In particular, in healthy brain, microglia cells seems to not express CB2, whereas this receptor is
undoubtedly expressed when the microglia are activated by inflammation (Stella, 2004; Yiangou et al., 2006; Carlisle et al., 2002; Stella, 2004; Cabral et al., 2008). The activation of CB2 receptor modulates the immune cell migration
such as neutrophils, macrophages, NK and B cells (Patel, 2010) and inhibits the inflammatory microglial response, determining a decrease of the cytokine release both outside and within the brain (Howlett, 2002). Briefly, cannabinoids may inhibit the production of tumor necrosis factor (TNF)-α, interleukin (IL)-1β and the p40 subunit of IL-12 and IL-23 by microglia and macrophages (Correa, 2009) and T cells proliferation (Patel, 2010) and may alter the cytokine profile from a T helper (Th)1 to a (Th)2 phenotype (Klein, 2005; Jean-Gilles, 2010). However, CB1 receptor has also been related to neuroinflammation regulation.
16
terminals leads to inhibition of glutamate release, limiting excitotoxic damage and thus exerting a direct neuroprotective effect (Zoppi et al., 2011).
Thus, due to a myriad of neuro-protective, neuroinflammatory and anti-oxidant actions, CB2 receptor agonists have been cogitated as possible
therapeutic agents for neurodegenerative disorders that combine inflammatory responses, as Alzheimer’s Disease (AD), Multiple Sclerosis (MS), Huntington and Parkinson Diseases (Saito et al, 2012). In fact, hyperactive microglia is a common feature of these diseases and secretes a number of pro- and anti-inflammatory cytokines, chemokines, glutamate, prostanoids, neurotrophic factors, and a range of free radicals that provide a milieu for oxidative stress. A promising medicine in the treatment of inflammation induced by microglial hyperactivation is the cannabinol (CBD), a phytocannabinoid which has no psychotropic activity (Booz et al., 2011).
Alzheimer’s disease (AD)
AD is a multifactorial age-related neurodegenerative condition characterized by progressive cognitive decline as a result of chronic synaptic loss and neuronal death. It is the most common forms of dementia (50-70% of dementia cases). The neuropathological hallmarks of AD are the presence in the brain of extracellular senile plaques and intracellular neurofibrillary tangles, as well as cognitive decline and memory deficits (Schaeffer et al., 2011; Cabral and Griffin-Thomas, 2012). Briefly, senile plaques mainly consist of fibrils of 39-42(43) amino acid β-amyloid (Aβ) peptide that are surrounded by dystrophic neurites and reactive glial cells (Schaeffer et al., 2011). The Aβ peptide itself is derived from the processing of a larger precursor protein known as the amyloid precursor protein (APP). The dysfunction of APP metabolism and the consequent accumulation of Aβ peptides and their aggregation in the form of senile plaques in the brain parenchyma of individuals with AD, have been considered crucial for neurodegeneration in the disease. This is the so-called
17
“amyloid cascade hypothesis”. Substantial evidence supports that the formation of these plaques is connected with the appearance of activated microglia that induce an inflammatory response in the central nervous system.
As AD is a neurodegenerative cognitive disorder with an inflammatory component, the cannabinoid system, which influences both the immune system and cognition, may have relevance in the treatment of AD. Ramirez and collegues have been reported that CB2 agonist can be neuroprotective in AD by
inhibiting the activation of microglia (Ramirez et al., 2005) induced by amyloid plaques consisting of extracellular aggregates of amyloid β (Aβ) peptides (Dickson, 1997). Recently, it was indicated that the CB1/CB2 agonist CP55940
and the CB2 agonist JWH-015 protect and rescue peripheral blood lymphocytes
from Aβ and H2O2-induced apoptosis by a receptor-independent pathway
(Velez-Pardo and Del Rio, 2006). Moreover, a CB1 receptor antagonist
prevented the amnesia induced by Aβ peptides, in a mouse model of AD, indicating that local production of eCBs in AD contributes to cognitive impairment (Mazzola et al., 2003). This study suggests that effects of eCBs on cognition could be important in AD where there is already cognitive impairment loosely associated with synapse loss but not absolutely linked to any distinct neuropathology (Koppel and Davies, 2008). There is growing evidence that local concentrations of eCBs can explain some of the phenomenology of memory impairment in AD. Advanced researches are necessary to determine what role eCBs play in management cognition and AD.
Multiple Sclerosis (MS)
Multiple sclerosis is a chronic disease affecting the CNS, which is characterized by inflammatory demyelinating and neurodegenerative features. The CNS infiltration of autoreactive T cells determine the degeneration of the myelin sheath that covers axons (Kieseier et al., 1999; Anthony et al., 2000). This causes an inflammatory process that stimulates other immune cells, such as B
18
cells and microglia, that together with T cells secrete proinflammatory mediators and antibodies, breakdown of the BBB, activation of macrophages, and production of “cytotoxic” proteins such as metalloproteinases (Friese and Fugger, 2005; Cabral and Griffin-Thomas, 2012). When myelin is lost, the axons can no longer effectively conduct signals. The term of multiple sclerosis is referred to scars (scleroses-better known as plaques or lesions) particularly in the white matter of the brain and spinal cord, which is mainly composed of myelin (Compston and Coles, 2002). Although much is known about the mechanisms involved in the disease process, the cause remains unknown.
Both CB1 and CB2 receptor expressing cells have been identified around plaques
in human MS (Yiangou et al., 2006; Benito et al., 2007). CB1 receptor is
expressed in cortical neurons, oligodendrocytes, macrophages, whereas a great CB2 expression has been localized to plaque-associated microglia and
macrophages, nearby astrocytes, and perivascular T cells (Benito et al., 2007). For these reasons, many studies have aimed at assess of effects of cannabinoids on MS, and the role of cannabinoid receptors in this process. Several experimental models have shown that the activation of cannabinoid receptors in the inflammatory demyelinative process characterizing MS may cause a neuroprotective effect through a CB1 receptor-mediated inhibition of
excitotoxicity and through a CB2 receptor-mediated inhibition of
neuroinflammation. In particular, in most of these studies, it was used a mouse model: the Experimental Autoimmune Encephalomyelitis (EAE) model, which exhibits a CD4 + T lymphocyte-mediated autoimmune disease (Racke, 2001). In the EAE model, Δ9-THC efficiently inhibits neurodegeneration and reduce the associated induced elevated level of glutamate in cerebrospinal fluid (Fujiwara and Egashira, 2004).
Regarding CB2 receptor, mRNA expression and protein internalization of this
receptor were found upregulated in activated microglia of mice experiencing EAE, suggesting the involvement of CB2 during this disease (Ehrhart et al.,
19
ameliorates EAE and diminishes cell infiltration of the spinal cord. Moreover, WIN55212-2 induce encephalitogenic T cell apoptosis through a mechanism in which the CB2 was partially involved (Sanchez et al., 2006). Recently,
Palazuelos and collegues have been proposed a protective role for CB2 receptor
in EAE pathology by targeting myeloid progenitor trafficking and its contribution to microglial activation in the CNS (Palazuelos et al., 2008). Another mouse model for human MS is obtained using Theiler's murine encephalomyelitis virus (TMEV), a natural mouse pathogen that induces a chronic, CD4+ T cell-mediated demyelinating disease with a clinical course and histopathology similar to that of chronic progressive MS. In this model, the treatment of mice with the synthetic cannabinoids WIN55212-2, ACEA (a CB1
-selective agonist) and JWH-015 (a CB2-selective agonist)improved neurological
deficits, concomitant with reduced microglial activation and T-lymphocyte infiltration (Arevalo-Martin et al., 2003). Moreover, the cannabinoid HU-210 was shown to ameliorate symptomology that was accompanied by a reduction of axonal damage. It was also observed that HU-210-mediated reduction in AMPA-induced excitotoxicity in vivo and in vitro was found to be linked to CB1
and CB2. Finally, it was shown that anandamide, through CB1 receptor
activation, inhibits the expression of Theiler’s virus-induced vascular cell adhesion molecule (VCAM)-1, an endothelial receptor that plays a key role in leukocyte transmigration in multiple sclerosis (Engelhardt et al., 2005; Greenwood, 2011).
20
Figure 6. Role of CB1 and CB2 receptors in the pathogenic events of MS(A) and ALS (B)
(Rossi et al., 2010).
Amyotrophic Lateral Sclerosis (ASL)
ASL is the third most common neurodegenerative disease characterized by an inflammatory component. It results in a progressive degeneration of motor neurons in the cortex, brainstem and spinal cord, leading to muscle wasting, weakness, and spasticity that progresses to complete paralysis (Cabral and Griffin-Thomas, 2012).
The etiology of ALS is incompletely understood but about 10% of the cases are familial and approximately the 20% of them, have mutations in the gene encoding for the antioxidant enzyme Cu/Zn- superoxide dismutase (SOD1) (Rossi et al., 2010). However, the pathological degeneration of motor neurons is well known and it has been related to several mechanisms, including neurofilament accumulation, excitotoxicity, oxidative stress, and neuroinflammation with microglial activation and release of proinflammatory
21
cytokines, prostaglandins, and nitric oxide (Babu et al., 2008; Rossi et al., 2010). All these pathological events are potentially modulated by the endocannabinoid system.
An animal model of familial ALS is the mutant SOD1 transgenic (i.e., SOD1G93A) mouse that develops clinical symptoms similar to those observed in humans. In this mouse model, it has been observed that the levels of the endocannabinoids (AEA and 2-AG) are increase in the spinal cord and are up-regulated with disease progression (Bilsland et al., 2006). This up-regulation might represent a neuroprotective response, not robust enough to counteract disease progression (Rossi et al., 2010). Motoneurons subjected to hyperstimulation of glutamate receptors may constitute an important source of endocannabinoids during excitotoxic processes since they are synthesized and released by neurons in an activity-dependent manner. Even CB2 receptor is
dramatically upregulated in the spinal cord of G93A-SOD1 mice, in a fashion that paralleled disease progression (Shoemaker et al., 2007). It was also reported, that daily injections of the CB2 agonist AM-1241, in G93A- SOD1
mice, initiated at onset of symptoms determined an increase the survival interval after disease onset by 56%. The stimulation of CB2 receptors exerts an
anti-inflammatory and neuroprotective role by reducing microglial activation and the release of proinflammatory cytokines and free radicals from microglia thus limiting motoneuron damage. There is also evidence that genetic CB1 receptor
ablation has beneficial effects in G93A SOD1 mice by extending their life span, consistently with the proposed involvement of CB1 receptor sensitization in ALS
progression (Bilsland et al., 2006). The glutamate and GABA transmissions are found to be remarkably potentiated in striatal neurons of SOD1 mice after the treatment with the selective CB1 receptor agonist HU-210.
Collectively, the results suggested that CB2 receptor agonists or CB1 receptor
22
Cannabinoid CB
2Receptor Ligands
This paragraph summarizes the main features of the structure that bind the cannabinoid CB2 receptor. Some of these molecules are frequently used as
pharmacological tools in cannabinoid research (for reviews see Howlett et al., 2002; Szabo, 2008; Pertwee et al., 2010).
Figure 7. Most known cannabinoid CB2 receptor ligands.
CB1 and CB2 Receptors Agonists
Several cannabinoid receptor agonists possess similar affinities for CB1 and CB2
receptors. When classified according to their chemical structures, these agonists fall essentially into four main groups: classical, nonclassical, aminoalkylindole, and eicosanoid.
o Classical Cannabinoids. The classical group consists of ABC-tricyclic dibenzopyran derivatives that are either compounds occurring naturally in the plant, Cannabis sativa, or synthetic analogs of these compounds. The most important compounds belonging to this class are the ∆9-THC, ∆8-THC, 11-hydroxy-∆8-THC-dimethylheptyl (HU-210) and the
desacetyl-L-23
nantradol. The first two are psychotropic plant cannabinoids wheras the desacetyl-L-nantradol and HU-210 are synthetic cannabinoids. HU-210 displays high affinity for CB1 and CB2 receptors and also high potency and
relative intrinsic activity as a cannabinoid receptor agonist. These properties are all thought to result mainly from the presence of its dimethylheptyl side chain. ∆9-THC possesses significantly lower CB1 and
CB2 affinity than HU-210 and lower relative intrinsic activity at these
receptors, an indication that ∆9-THC is a cannabinoid receptor partial agonist. ∆8-THC has CB1 and CB2 affinity and intrinsic activity that are
similar to those of ∆9-THC. O OH O OH ∆9-THC ∆8-THC Ki CB1: 41 nM Ki CB1: 44 nM Ki CB2: 36 nM Ki CB2: 44 nM O OH OH N H OH O OH HU-210 DESACETYL-L-NANTRADOL Ki CB1: 0.7 nM Ki CB2: 0.2 nM
o Non Classical Cannabinoid. This group contains bicyclic and tricyclic analogs of ∆9-THC that lack a pyran ring. A well known member of this group is (-)-cis-3-[2- hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol (CP55940), which has become one of the
24
major cannabinoid agonists. CP55940 behaves as a full agonist for both receptor types. OH HO H OH H CP55940 Ki CB1: 0.6 nM Ki CB2: 0.7 nM
o Aminoalkylindole Group.These compounds have structures that differ markedly from those of both classical and nonclassical cannabinoids. The best known member of this group is R-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1
naphthalenylmethanone mesylate (R-(+)-WIN55212), whereas its S-(-)-enantiomer, WIN55212-3, lacks activity both in vivo and in vitro. This cannabinoid agonist displays CP55940-and HU-210-like relative intrinsic activity at both CB1 and CB2 receptors. However, unlike HU-210 and
CP55940, it has been found in some investigations to possess slightly higher CB2 than CB1 affinity. There is evidence that R-(+)-WIN55212
binds differently to the cannabinoid receptors than classical or nonclassical cannabinoids. O N O N O WIN55212-2 Ki CB1: 1.9 nM Ki CB2: 0.3 nM
25
o Eicosanoid Group. These compounds possess structures quite unlike those of classical, nonclassical, or aminoalkylindole cannabinoids. The endocannabinoids AEA and 2-AG are the most representive members of this group. Like ∆9-THC, anandamide possess a CB1 and CB2 receptor
partial agonist activity and displays lower relative intrinsic activity for CB2
than for CB1. This eicosanoid does, however, have slightly lower receptor
affinity for CB2 than for CB1 and consequently displays less affinity for
the CB2 receptor than ∆9-THC. 2-AG also has slightly less receptor
affinity for CB2 than for CB1. It seems to have lower CB1 receptor potency
than CP55940 but higher CB1 and CB2 receptor potency than anandamide
and higher CB1 receptor relative intrinsic activity than anandamide or
CP55940.
CB2-Selective Cannabinoid Receptor Agonists
While the most CB1-selective agonists are eicosanoids, the CB2-selective
agonists are chemically more heterogeneous: classical cannabinoids JWH-139,
3-(1,1-dimethylpropyl)-6,6,9-trimethyl-6a,7,10,10a-tetrahydro-6H-benzo[c]chromene; JWH-133, 3-(1,1-dimethylbutyl)-6,6,9-trimethyl-6a,7,10,10a-tetrahydro-6Hbenzo[c] chromene; synthetic nonclassical cannabinoid HU-308, 4-[4-(1,1-Dimethylheptyl)-2,6-dimethoxyphenyl]-6,6-dimethylbicyclo[3.1.1]hept-2-ene-2-methanol; aminoalkylindoles JWH-015; (2-methyl-1-propyl-1H-indol-3-yl)-1 naphthalenylmethanone; AM1241, R-3-(2-iodo-5-nitrobenzoyl)-1-methyl-2-piperidinylmethyl)-1H-indole.
26 N O N O N NO2 JWH-015 AM1241 Ki CB1: 336 nM Ki CB1: 580 nM Ki CB2: 14 nM Ki CB2: 7 nM O O JWH-139 JWH-133 Ki CB1: 2290 nM Ki CB1: 677 nM Ki CB2: 14 n Ki CB2: 3 nM O OH O HU-308 Ki CB1: > 10000 nM Ki CB2: 23 nM
Potent CB2-selective compounds with interesting analgesic, immunomodulatory
and anti-cancer activities are also 1,8-naphthyridine, quinoline and quinolone 3-carboxylic acid and amide derivatives (Manera et al., 2007; Pasquini et al., 2008), N-alkyl isatin acylhydrazone derivatives (Diaz et al., 2009) and some sulphamoyl benzamides (Goodman et al., 2009).
27
CB2-Selective Antagonists/Inverse Agonist
The most potent compounds that block the CB2 than CB1 receptors are
[6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)
methanone (6-iodopravadoline) (AM630) and the diarylpyrazole N-[(1S)-endo-1,3,3-trimethyl bicyclo[2.2.1]heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)pyrazole-3-carboxamide (SR144528). They display much higher affinity for CB2 than for CB1 receptors and block agonist-induced CB2 receptor
activation in a competitive manner. Both these compounds are thought to be CB2 receptor inverse agonists rather than neutral antagonists, because when
administered by themselves, they can produce inverse cannabimimetic effects in CB2 receptor-expressing tissues. Other CB2-selective cannabinoid receptor
antagonists/inverse agonists include
N-(1,3-benzodioxol-5-ylmethyl)-1,2-dihydro-7-methoxy-2-oxo-8-(pentyloxy)-3-quinoline carboxamide (JTE907). A neutral antagonist that selectively targets the CB2 receptor has not yet been
developed. N N Cl NH O N I N O O O SR144528 AM630 Ki CB1: 437 nM Ki CB1: 5152 nM Ki CB2: 0.6 nM Ki CB2: 31 nM O O H N O NH O O O JTE907 Ki CB1: 2370 nM Ki CB2: 36 nM
28
Introduction of Experimental Section
Cannabinoids are a unique family of terpenophenolic active constituents of
Cannabis sativa; ∆9-tetrahydrocannabinol (THC) is the most relevant owing to
its psychoactive effects and a wide variety of pharmacological effects (Velasco et al., 2012). The isolation and characterization of THC (Gaoni and Mechoulam, 1964) allowed for the identification of two distinct cannabinoid receptors, named CB1 and CB2 that have been cloned and characterized from mammalian
tissues (Matsuda et al., 1990; Munro et al., 1993). The CB1 receptor is
abundantly expressed in the central nervous system (CNS) with the highest densities in the hippocampus, cerebellum, and striatum (Ameri, 1999). Locations outside the brain have also been indicated, including adipose tissue, liver, muscle, the gastrointestinal tract, pancreas, urinary bladder, lung, heart, adrenal gland, testis, uterus and prostate (Gérard et al., 1991; Wenger et al., 2001; Das et al., 1995; Galiègue et al., 1995). In contrast, CB2 has been reported
as being limited essentially to the cells associated with the immune system, such as spleen, thymus, and tonsils (Munro et al., 1993) but it also found in low concentration in the brain.
Since the discovery of the cannabinoid receptors and their endogenous ligands, numerous studies implicate the endocannabinoid system in several physiological and pathological processes including cancer, appetite, fertility, memory, neuropathic and inflammatory pain, obesity, neurodegenerative diseases etc. (reviewed in Battista et al., 2012). At the present time, CB2 receptor has gained
29
attention as potential target for its immunoregulatory effects. Recent advances suggest a role for the CB2 receptors within the nervous system, particularly in
inflammatory conditions such as neurodegenerative diseases (Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, multiple sclerosis, etc.), given CB2 receptor up-regulated in the brain under these conditions and disease
states. This finding is supported by results obtained by Cabral and co-workers, from observing the pattern of expression of CB2 receptor during microglia
differentiation using an in vitro model of multistep activation (Carlisle et al., 2002; Cabral and Marciano-Cabral, 2005; Benito et. al, 2003). Additional data suggest that CB2-selective agonists show promise for suppressing inflammatory
and neuropathic pain states. Behavioral, electrophysiological and neurochemical studies all support a role for CB2 receptor activation in modulating inflammatory
nociception. Moreover, recent reviews have focused on covering the evidence for the functional neuronal presence and the emerging role of CB2 receptor in
neuropsychiatric disorders (Onaivi et al., 2009; Roche and Finn, 2010). Finally, CB2 is over-expressed in several tumor cells and various in vitro studies and in
animal models showed that activation of the CB2 receptor induces apoptosis,
inhibits tumour growth and inhibits neo-angiogenesis (reviewed in Velasco et al., 2012; Cianchi et al., 2008).
The effectiveness of selective CB2 agonists as neuroprotective and anticancer
agents prompted us to report our efforts in this field. Our aim was to identify a class of selective CB2 agonist as potential drugs devoid of the psychotropic
side-effects associated with the CB1 receptor.
Within a research program to identify novel CB2 selective ligands, our research
group previously described the synthesis, binding activity and molecular modeling studies of a series of 1,8-naphthyridin-4(1H)-on-3-carboxamide derivatives with general structure A. These compounds generally exhibited a remarkable CB2 affinity, with a Ki value in the nanomolar range. This affinity
was accompanied by a high selectivity with respect to CB1 receptor. Moreover,
30
indicated that these derivatives behaved as CB1 and CB2 receptor agonists
(Manera et.al., 2006; Manera et al., 2007).
N N N H O R2 R1 O A R3
Furthermore one of these compounds, which showed high CB2 receptor affinity
with a good CB2 versus CB1 selectivity, exhibited in vivo antinociceptive effects
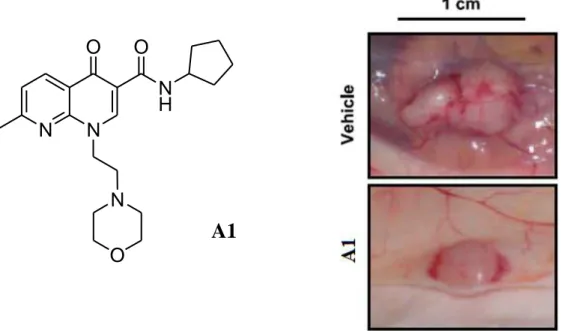
(Manera et al., 2007). Finally another compound of this series was evaluated for the antitumor effects in experimental models of colon cancer. The tumor was generated in immunodeficient mice by subcutaneous injection of DLD-1 cells,that showed a high expression of CB2 receptors. As shown in Figure 8,
peritumoral treatment with 1,8-naphthyridine derivative A1 significantly reduced the growth of the established colon tumors (Cianchi et al., 2008).
Figure 8. Photographs of a representative vehicle-treated and A1-treated tumor.
In order to investigate possible modifications of the 1,8-naphthyridin-4(1H)-one central scaffold, the compound
N-cyclohexyl-1-(2-morpholin-4-ylethyl)-1,8-N N O N H O N O A1
31
naphthyridin-4(1H)-on-3-carboxamide (A2, see Figure 9) (Ki CB1 > 1000 nM; Ki CB2=48.6 nM) was chosen as a lead for the development of new derivatives (Manera et al., 2009). The superimposition between this reference compound and the N-cyclohexyl-1-(2-morpholin-4-ylethyl)-1,8-naphthyridin-2(1H)-on-3-carboxamide (B1) analogue encouraged further experimental studies. As shown in Figure 10 the superimposition between the two compounds highlighted a satisfactory overlap of the structural features deemed important for the CB2
affinity, i.e., the lipophilic central core, the cyclohexyl, and the morpholine substituents. On the basis of these results, a series of 1,8-naphthyridin-2(1H)-on-3-carboxamide derivatives (B1-B12) were synthesized (Manera et al., 2009).
N N N H O O N O N N N H O N O O N N N H O R2 R1 O N N N H O O R1 R2 A B A2 B1
Figure 9. General structures of the naphthyridin-4(1H)-on-3-carboxamide (A) and 1,8-naphthyridin-2(1H)-on-3-carboxamide (B) derivatives and structures of compound A2 and B1.
32
Figure 10. 3D structure and solvent-excluded molecular surface of compound A2 (A), B1 (B), and their superimposition (C).
These compounds showed CB2 receptor affinity and selectivity that were higher
than corresponding 1,8-naphthyridin-4(1H)-on-3-carboxamide derivatives A previously studied(Manera et al., 2006) and, in some cases (i.e., compound B5), among the highest ever obtained for CB2 receptor ligands (Table 1). In
particular, the (p-fluorobenzyl)-1,8-naphthyridine derivatives B9 and B12-cis exhibited the highest affinity, with Ki values of 0.7 nM. The derivatives B5,
B11, B11-cis, B12 and B12-cis showed very remarkable selectivity with a
Ki(CB1)/Ki(CB2) ratio >200. Interestingly, the
1,8-naphthyridin-2(1H)-on-3-carboxamide derivatives B11 and B12 showed a stereoselectivity for the binding to the CB2 receptor. In fact, B11-cis and B12-cis showed 7-fold and 13-fold
increases in their affinity for the CB2 receptor when compared with the
corresponding B11-trans and B12-trans, respectively. Furthermore, the concentration-dependent activity shown by derivative B11 in human basophils and in Jurkat cells strongly suggests that this compound and the other 1,8-naphthyridin-2(1H)-on-3-carboxamides possess agonist properties at CB2
receptor (Manera et al., 2009). These results indicate that 1,8-naphthyridin-2(1H)-on-3-carboxamides represent new scaffolds that may be suitable for the development of new potent and selective CB2 receptor agonists to be used in
33
Table 1. Radioligand Binding Data of Compounds B1-B12a
Compd. R1 R2 Ki CB1 (nM)b KiCB2 (nM)c KiCB1/ KiCB2 B1 ethylmorpholino o. cyclohexyl 560 7.9 71 B2 benzyl cyclohexyl 560 31 18 B3 p–fluorbenzyl cyclohexyl 56 2.2 25 B4 n–butyl cyclohexyl 5600 70 80 B5 ethylmorpholino 4–methylcyclohexyl 1000 1.9 526 B6 benzyl phenethyl NT NT NT B7 benzyl 4–fluorophenethyl 6947 69.8 100 B8 benzyl cycloheptyl 960 22 44 B9 p–fluorbenzyl cycloheptyl 9.6 0.7 14 B10 ethylmorpholino cycloheptyl 18 3 6 B11 benzyl 4–methylcyclohexyl 1600 7.8 205 B11trans benzyl 4–methylcyclohexyl 5255 39.8 132 B11cis benzyl 4–methylcyclohexyl 1519 5.8 262
B12 p–fluorbenzyl 4–methylcyclohexyl 200 0.9 222
B12trans p–fluorbenzyl 4–methylcyclohexyl 300 9.0 33 B12cis p–fluorbenzyl 4–methylcyclohexyl 200 0.7 286
SR144528 437 0.6 728
JWH-133 677 3 226
a Data represent mean values for at least three separate experiments performed in duplicate and are
expressed as Ki (nM) for CB1 and CB2 binding assays. Values of standard error of the mean (SEM) are not shown for the sake of clarity and were never higher than 5% of the means. b Affinity of compounds
for CB1 receptor was evaluated using membranes from HEK-293 cells transfected with CB1 receptor
and [3H]CP55,940. c Affinity of compounds for CB2 receptor was evaluated using membranes from
34
Starting from these achievements, during my PhD I have developed three themes:
1. Identification of 1,8-naphthyridin-2(1H)-on-3-carboxamide derivatives as new potent and selective ligands for cannabinoid CB2 receptor: synthesis,
pharmacological evaluation and docking studies.
2. Development of 1,8-naphthyridin-2(1H)-one derivatives for PET imaging of cannabinoid CB2 receptor.
3. Rational design, synthesis and pharmacological evaluation of new cannabinoid receptor ligands: an investigation of the 1,8-naphthyridin-2(1H)-one scaffold.
35
1. Identification of 1,8-naphthyridin-2(1H)-on-3-carboxamide derivatives as new potent and selective ligands for cannabinoid CB2 receptor: synthesis, pharmacological evaluation and docking studies
In an effort to develop improved naphthyridine-based CB2 cannabinoid receptor
ligands and also to develop structure activity relationship (SAR) for both the CB1 and CB2 receptors, I synthesized a number of additional
1,8-naphthyridine-2(1H)-on-3-carboxamide derivatives (B13-B35) in which the central naphthyridine scaffold has been variously functionalized with new various substituents in position 1 and/or 6. The choice of 4-methylcyclohexyl carboxamide group in position 3 is based on the observation of the binding results obtained for derivatives A and B previously studied.
First step was to explore the binding pocket of the human CB2 receptor by
introduction of several groups with different steric and lipophilic properties in position N-1 of the naphthyridine nucleus. The synthesis of compounds
B13-B22 was achieved in a three step procedure and is depicted in Scheme 1.
Heating of 2-aminopyridin-3-carboxaldheyde with diethylmalonate and in presence of piperidine in EtOH at reflux for 24 h afforded the 1,8-naphthyridin-2(1H)-on-3-carboxylic acid ethyl ester (1) (Manera et al., 2009). Then, the treatment of ethyl ester 1 with the 4-methylcyclohexylamine in sealed tube for 24 h at 150°C provided the desired carboxamide 2. N1-alkylation of
R3: H R3: thiophenyl R3: furyl R3: p-methoxyphenyl R3: p-fluorophenyl R1: CH2(CH2)nCH2COOH R1: CH2(CH2)nCH2COOEt R1: CH2(CH2)nCH2CH3 R1: ethylmorpholino R1: p-fluorobenzyl R2: 4-methylcyclohexyl
36
carboxamide 2 in anhydrous DMF with the suitable reagent in the presence of cesium carbonate at 50°C for 12 h afforded the desired compounds B13-B22.
Scheme 1 N NH2 H O N H O O O N N H O N H O N i ii iii N O N H O N R1 1 2 B13-B22
Reagents and conditions: (i) diethylmalonate, piperidine, EtOH, reflux, 12 h; (ii) 4-methylcyclohexylamine, 150°C, 24 h; (iii) Cs2CO3, R1Cl or R1Br, DMF, 50°C, 12 h.
Subsequently, the carboxylic acid derivatives (B23-B25) were synthesized from the corresponding esters (B20-B22) by alkaline hydrolysis and then acidification (Scheme 2). B13 R1: CH2CH2CH2CH3 B14 R1: CH2CH2CH2CH2CH3 B15 R1: CH2CH2OH B16 R1:CH2CH2CH2OH B17 R1:CH2(CH2)3OH B18 R1:CH2(CH2)4OH B19 R1:CH2(CH2)5OH B20 R1:CH2CH2COOEt B21 R1:CH2CH2CH2COOEt B22 R1:CH2(CH2)3COOMe
37 Scheme 2 N O N H O N COOR n N O N H O N COOH n NaOH aq. 10% 110°C, 5 h
The second strategy to obtain compounds that could provide additional interactions with the CB2 receptor binding site compared to compounds of type
B, I synthesized the compounds B26-B35 which are characterized by various
lipophilic groups in position 6 of the 1,8-naphthyridine core. Substituents in position 1, p-fluorobenzyl and ethylmorpholino, have been selected on the basis of the binding results obtained for the derivatives B1-B12 on CB2 receptor
(Manera et al., 2009).
The synthetic route to obtain the 6-substitued-1,8-naphthyridin-2(1H)-on-3-carboxamide derivatives B26-B35 is outlined in Scheme 3. The 2-aminopyridin-3-carboxaldheyde was treated with Bromine in glacial acetic acid at room temperature for 24 h to obtain the corresponding 6-bromo derivative 3, which was refluxed with diethylmalonate and in presence of piperidine in EtOH for 24 h to afford the 6-bromo-1,8-naphthyridin-2(1H)-on-3-carboxylic acid ethyl ester (4). Initially to obtain the derivative 4, the 1,8-naphthyridin-2(1H)-on-3-carboxylic acid ethyl ester (1) was treated with Br2 in the same conditions above
reported, but the yield was very low.
B20 (n=1) R: Et B21 (n=2) R: Et B22 (n=3) R: Me B23 (n=1) B24 (n=2) B25 (n=3)